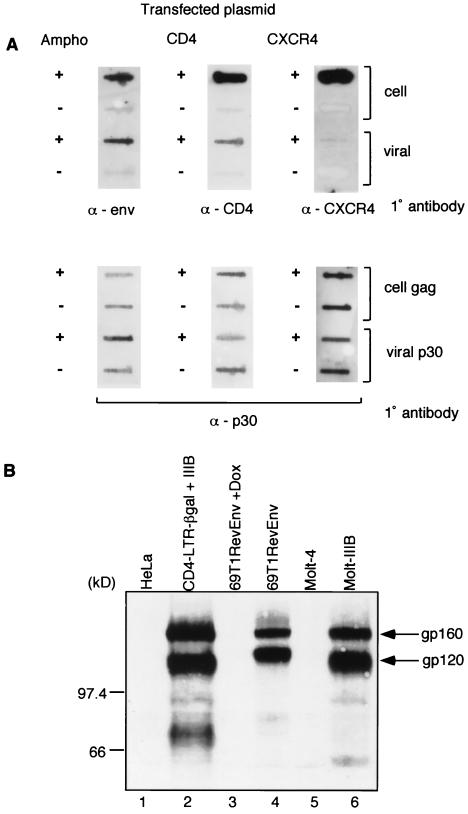
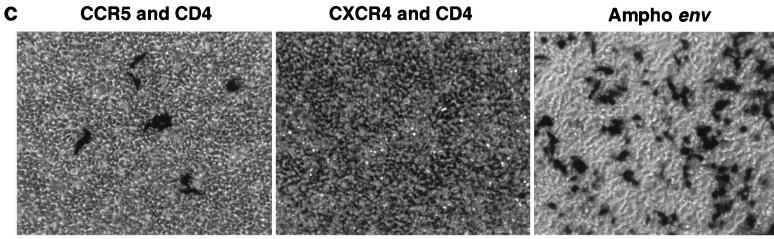
FIG. 2.
(A) Immunoblot analysis of amphotropic envelope, CD4, and CXCR4 proteins in transfected cells and viral particles. Twenty micrograms of expression constructs for the amphotropic envelope (SV-A-MLV-env) (Ampho) (21), CD4 (CMX-CD4, a gift from Didier Trono), or CXCR4 (pc.Fusin) (9) were transfected into 5 × 105 293gp/lacZ cells, according to the method of Chen and Okayama (4). Mock-transfected cells (−) were similarly treated, but no plasmid was transfected. After overnight incubation, the medium was changed, and the vector was harvested 48 h later. The cells were collected and lysed in 1 ml of lysis buffer (20 mM Tris-HCl [pH 8.0], 1 mM CaCl2, 150 mM NaCl, 1% Triton), and the cell extract (1 μl for the amphotropic envelope, CD4, and loading controls; 10 μl for CXCR4) was transferred to a nitrocellulose membrane for immunoblot analysis. The supernatant was filtered through a 0.45-μm-pore-size filter, the viral particles were pelleted through a 20% sucrose cushion by ultracentrifugation (90,000 × g for 1.5 h) and resuspended in 1 ml of lysis buffer, and the viral extract (1 μl for the amphotropic envelope, CD4, and loading controls; 10 μl for CXCR4) was transferred to a nitrocellulose membrane for immunoblot analysis. Primary (1°) antibodies used were goat anti-Raushers gp69/71 (National Cancer Institute/Biological Carcinogenesis Branch repository) (α-env), rabbit anti-human CD4 (8) (α-CD4), and mouse anti-human CXCR4 (monoclonal antibody 4G10, a gift from Edward Berger) (α-CXCR4). Secondary antibodies were rabbit anti-goat immunoglobulin G (IgG)-horseradish peroxidase (HRP) conjugate (Pierce), donkey anti-rabbit IgG-HRP conjugate (Amersham), and rabbit anti-mouse IgG-HRP (Pierce). The secondary antibodies were detected by using the ECL Western blotting detection system (Amersham). The loading controls were probed with a goat anti-Raushers p30 antibody (NCI/BCB repository). The Gag polyprotein (for cell pellets) and p30 (for viral pellets) are shown as loading controls in the bottom panel. (B) Immunoblot analysis of gp120 expression. The following cell extracts (100 μg) were used: uninfected HeLa cells, HeLa-CD4-LTR/β-gal cells infected with T-cell-tropic HIV-1 IIIB, 69T1RevEnv cells grown in the presence of Dox (1 μg/ml), 69T1RevEnv cells, uninfected Molt-4 cells, and the HIV-1 IIIB-infected cell line Molt-IIIB. The primary antibody was sheep anti-gp120 (from Michael Phelan), and the secondary antibody was donkey anti-sheep IgG-HRP conjugate (Sigma). The slower-migrating band is the unprocessed env gene product gp160. (C) CCR5 incorporation into a Moloney MLV vector. 293 cells were transfected with 15 μg of an expression plasmid for macrophage-tropic gp120 (pSV-JRFL-env) and 5 μg of an expression plasmid for Rev. The cells were infected 48 h later with a β-Gal-transducing retroviral vector. The plasmid combinations that were transfected into 293/lacZ cells to pseudotype and generate the β-Gal vector are shown at the top.