Abstract
IMPORTANCE:
Microvascular autoregulation (MA) maintains adequate tissue perfusion over a range of arterial blood pressure (ABP) and is frequently impaired in critical illness. MA has been studied in the brain to derive personalized hemodynamic targets after brain injury. The ability to measure MA in other organs is not known, which may inform individualized management during shock.
OBJECTIVES:
This study determines the feasibility of measuring MA in skeletal muscle using near-infrared spectroscopy (NIRS) as a marker of tissue perfusion, the derivation of optimal mean arterial pressure (MAPopt), and comparison with indices from the brain.
DESIGN:
Prospective observational study.
SETTING:
Medical and surgical ICU in a tertiary academic hospital.
PARTICIPANTS:
Adult critically ill patients requiring vasoactive support on the first day of ICU admission.
MAIN OUTCOMES AND MEASURES:
Fifteen critically ill patients were enrolled. NIRS was applied simultaneously to skeletal muscle (brachioradialis) and brain (frontal cortex) while ABP was measured continuously via invasive catheter. MA correlation indices were calculated between ABP and NIRS from skeletal muscle total hemoglobin (MVx), muscle tissue saturation index (MOx), brain total hemoglobin (THx), and brain tissue saturation index (COx). Curve fitting algorithms derive the MAP with the lowest correlation index value, which is the MAPopt.
RESULTS:
MAPopt values were successfully calculated for each correlation index for all patients and were frequently (77%) above 65 mm Hg. For all correlation indices, median time was substantially above impaired MA threshold (24.5–34.9%) and below target MAPopt (9.0–78.6%). Muscle and brain MAPopt show moderate correlation (MVx–THx r = 0.76, p < 0.001; MOx–COx r = 0.69, p = 0.005), with a median difference of –1.27 mm Hg (–9.85 to –0.18 mm Hg) and 0.05 mm Hg (–7.05 to 2.68 mm Hg).
CONCLUSIONS AND RELEVANCE:
This study demonstrates, for the first time, the feasibility of calculating MA indices and MAPopt in skeletal muscle using NIRS. Future studies should explore the association between impaired skeletal muscle MA, ICU outcomes, and organ-specific differences in MA and MAPopt thresholds.
Keywords: critical care, hemodynamic monitoring, microcirculation, near-infrared spectroscopy, skeletal muscle
KEY POINTS
Question: In critically ill patients requiring vasoactive support, can microvascular autoregulation (MA) indices be measured in skeletal muscle using near-infrared spectroscopy (NIRS), and can an optimal mean arterial pressure (MAPopt) be calculated that minimizes impairments in MA?
Findings: In this observational cohort of fifteen patients, skeletal muscle MA indices and MAPopt were successfully derived for each patient. Patients frequently had MA indices above a dysfunctional threshold, and blood pressure below their target MAPopt.
Meaning: This study describes, for the first time, the measurement of skeletal muscle MA and MAPopt using NIRS in critically ill patients, which may inform personalized management of patients with shock.
Hemodynamic monitoring in the ICU is used for the management of patients with circulatory failure and shock and has traditionally focused on systemic parameters such as blood pressure, cardiac output, and lactate (1). Often, these systemic parameters also serve as universal resuscitation targets, such as the minimum threshold of 65 mm Hg for mean arterial pressure (MAP) in hypotensive patients. Recently, these systemic hemodynamic parameters have been augmented with clinical markers of peripheral perfusion, such as capillary refill time (CRT) (2), which can be used as surrogate measurements for the status of the microcirculation (3) and applied to guide resuscitation (4). Although microvascular status and peripheral perfusion correlate better with ICU outcomes than systemic hemodynamics (5), knowledge gaps still exist regarding how they can be incorporated into routine clinical practice.
Microvascular autoregulation (MA) denotes the ability of the microcirculation to regulate blood flow over a range of systemic blood pressures to ensure adequate tissue perfusion and oxygen delivery; MA is frequently disrupted during critical illness and contributes to organ failure. This concept has been most widely studied in the field of neurocritical care, where cerebral MA has been measured as the correlation between blood pressure and invasive (e.g., intracranial pressure monitoring) and noninvasive (e.g., near-infrared spectroscopy [NIRS]) modalities (6). MA can be used to derive personalized hemodynamic targets such as optimal cerebral perfusion pressure (CPPopt) for management of brain injury (7–9), and impaired cerebral MA has been associated with poor outcomes in these patient populations (10–13).
Skeletal muscle is the largest organ by mass, the main site of action for vasoactive medications, and a main determinant of systemic vascular resistance; therefore, it serves as an important and accessible hemodynamic organ for evaluating peripheral tissue perfusion. Although the concept of blood flow regulation within skeletal muscle microcirculation is widely accepted with both preclinical foundations (14, 15) and applications within exercise physiology (16), the presence of MA in skeletal muscle has not been assessed in the critical care setting. The goal of this study is to determine the ability to measure MA in skeletal muscle using NIRS in the ICU, as well as calculating optimal MAP (MAPopt) from skeletal muscle-derived MA indices. We will also compare MAPopt values from skeletal muscle and brain to evaluate organ-specific hemodynamic discrepancies. These novel hemodynamic metrics from the peripheral microcirculation may inform personalized hemodynamic management of patients with shock (17), including individualized blood pressure targets (18).
METHODS
Study Details
This prospective observational cohort study was conducted at the Winnipeg Health Science Center Medical and Surgical ICUs from May 2022 to July 2023. All research practices and protocols were approved by the University of Manitoba, Biomedical Research Ethics Board HS25043 (September 15, 2021), and by the Shared Health Approval Committee SH2021-147 (November 21, 2021) for the study entitled “MIMICSS: Microvascular Monitoring in Circulatory Shock in Sepsis: Longitudinal Observational Cohort Study” in accordance with the Declaration of Helsinki; the study was registered at clinicaltrials.gov (NCT05985525). The study enrolled adult critically ill patients on the first day of ICU admission that required vasoactive medications to maintain MAP above 65 mm Hg; mechanical ventilation was an additional inclusion criterion to minimize movement artifacts and optimize data quality. Data on patient demographics, clinical history, and laboratory values were collected from the medical records. Monitoring was initiated with deferred consent; written informed consent was obtained from the patient or substitute decision-maker as soon as possible for all enrolled patients.
Microvascular Monitoring Using High-Resolution NIRS
In this study, high-resolution NIRS (hr-NIRS) was used to assess MA in critically ill patients. One device (PortaMon; Artinis Medical Systems, Elst, Netherlands) was positioned on the skeletal muscle (brachioradialis), whereas the other device (PortaLite; Artinis Medical Systems) was placed on the forehead 3–4 cm above the eyebrow (frontal cortex); the setup is shown in Supplementary Figure 1 (http://links.lww.com/CCX/B352). Differential path length (DPF) is set for the muscle at four, and DPF is set for the brain based on the age-recommended values from the manufacturer. These spectrometers record at high temporal resolution (10 Hz) and employ a spatially resolved spectroscopy method using three light transmitters and one receiver (30, 35, and 40 mm). Each transmitter-receiver pair measures the relative change in oxyhemoglobin and deoxyhemoglobin concentrations and the relative change in total hemoglobin (HbT) concentration; we used the 40 mm distance in our analysis to maximize tissue penetration depth. Using all three transmitter-receiver pairs enables the approximation of the tissue scattering coefficient and calculation of tissue saturation index (TSI), analogous to tissue oxygen saturation (Sto2, regional cerebral oxygen saturation) reported with similar NIRS systems. NIRS data are transmitted via Bluetooth to the NIRS software (OxySoft; Artinis Medical Systems). Arterial blood pressure (ABP) was measured via an invasive arterial catheter zeroed at the level of the right atrium, inserted by the clinical team as part of the standard of care, and acquired continuously with a DT9804 (Data Translation, Marlboro, MA) data acquisition module from the bedside ICU monitor at 100 Hz. All the NIRS and ABP signals were merged and synthesized in ICM+ (Cambridge Enterprises, Cambridge, United Kingdom) software for further processing. Before recording, the thickness of adipose tissue was measured over the brachioradialis using a standard ultrasound and linear probe (Sonosite, Toronto, ON, Canada) and the finger capillary refill test was conducted on each patient as previously described (4).
Microvascular Autoregulation and Derivation of Optimal Mean Arterial Pressure
Raw NIRS and ABP signals were manually inspected, and motion artifacts were removed using the ICM+ artifact cleaning tool. MA was determined based on the correlation between the NIRS signal and ABP, as has been described previously for cerebral NIRS (19). To minimize the influence of high-frequency components of respiratory and cardiac activities, as well as focus on the microvascular frequencies, a 10-second moving average was applied to the signals (9). Subsequently, the averaged ABP was designated as MAP. A Pearson correlation analysis was then conducted between MAP and each of the four NIRS signals within a 5-minute window, with updates every minute. These correlation coefficients are denoted as muscle HbT (MVx), brain HbT (THx), muscle TSI (MOx), and brain TSI (COx). This is a standard approach in similar studies to calculate autoregulation indices (9, 20, 21). Under normal conditions, correlation indices are presumed to have a low or negative value, reflecting intact MA; in disease conditions, these indices become strongly positive reflecting a pressure-passive system with a loss of MA (6).
MAPopt was determined using the four correlation indices listed above: MVx, THx, MOx, and COx. The procedure for calculating MAPopt was executed using ICM+ software. After preprocessing to achieve normal distribution, each index is grouped into MAP-based bins, and an error bar chart illustrates the relationship between the correlation index and MAP. Adjustments to the default ICM+ configuration can be made, such as reducing the exclusion threshold or modifying the width of the MAP bins. The algorithm aims to fit a curve to this plot, ideally forming a smooth U-shaped pattern. It follows specific criteria to ensure the quality of the curve fit, including the exclusion of outlier bins, representing at least 50% of all data points within the analyzed window period, and accounting for a minimum of 20 mm Hg of MAP fluctuation. If the criteria are met, the algorithm stops, returning the minimum of the fitted curve as the MAPopt value, which is the MAP with the best preserved MA. In some scenarios, only the ascending or descending sections of the curve may be available within the given MAP range, and the minimum value of these curves is reported as the MAPopt (20). Because of the relatively short length of our recording (hr), this algorithm was executed on a fixed window spanning the entire duration of each recording session for each patient, as described originally by Steiner et al (22) for the CPPopt method. However, we also explored newer methodologies, including the optimal flex window method (23), and with various window sizes (1–8 hr) and MAP bin widths (2–5 mm Hg).
Statistical Analysis
The statistical analysis was performed using the Prism software package (GraphPad Software, La Jolla, CA). Data are reported as mean ± sd or median (interquartile range [IQR]), where appropriate. Descriptive statistics for MA indices are reported with the median, IQR, and coefficient of variation over the course of the recording. Additionally, the time, percentage of total recording, and dose per hour where the MA correlation indices exceeded the predetermined threshold of 0.3 were quantified. This threshold has been widely used in previous research to identify high irregular covariation between systemic and microvascular regulation (13, 24–27). In addition to calculating the MAPopt for each MA index for each patient, the percentage of time spent below the MAPopt of each index was also computed. This metric may signify impaired MA, as previous studies have linked the percentage of time below the CPPopt to a higher risk of death in traumatic brain injury patients (28–31). The Pearson correlation was calculated to assess the association between MAPopt values derived from brain and muscle signals, and between HbT- and TSI-derived MAPopt, at a 5% level of significance.
RESULTS
Patient and Monitoring Characteristics
A total of 15 patients were included in this study, with demographics presented in Table 1. During the study period, 180 patients were screened, with a flow diagram of recruitment included in the Supplementary Material (http://links.lww.com/CCX/B352). The average duration of patient recording was 394 ± 229 minutes; following the removal of motion artifacts, the signals demonstrated an average duration of 329 ± 168 minutes. Among these participants, four of 15 patients (26.7%) were female. The mean ± sd age of the cohort was 63 ± 14 years, and ten of 15 patients (66.7%) had a diagnosis of sepsis. Ten of 15 patients (67%) had some form of preexisting cardiovascular comorbidity, including hypertension, diabetes, vascular disease, or heart failure. The median (IQR) norepinephrine dose was 0.13 µg/kg/min (0.08–0.17 µg/kg/min) during the recording period; nine of 15 patients (60%) were also on vasopressin (eight patients at 0.04 U/min, one patient at 0.02 U/min). Median (IQR) serum lactate levels were 1.8 mmol/L (1.45–3.00 mmol/L) and CRT was normal (< 3 s) for 12 of 15 patients (80%). Adipose tissue thickness overlying the brachioradialis muscle was 3.4 ± 1.0 mm (n = 13 patients, two patients with missing data). Sensors were placed on the forearm and forehead ipsilaterally in 11 patients and contralaterally in three patients (n = 14, one patient with missing data).
TABLE 1.
Demographics for the Patients in the Cohort
| Patient Demographic | n = 15 |
|---|---|
| Age (yr) | 63 ± 14 |
| Female | 4/15 |
| Sepsis | 10/15 |
| Norepinephrine dose (µg/kg/min) | 0.13 (0.08–0.17) |
| Vasopressin | 9/15 |
| Lactate (mmol/L) | 1.8 (1.45–3.00) |
| Acute Physiology and Chronic Health Evaluation II score | 19 (15.5–23) |
| Capillary refill time < 3 s | 12/15 |
| Mean arterial pressure (mm Hg) | 74 ± 3 |
| Heart rate | 90 ± 19 |
| Arterial blood pressure range (mm Hg) | 38 (29.5–46.0) |
Data are represented as mean ± sd, median (interquartile range), or proportion of the total cohort.
Impaired Microvascular Autoregulation in Critically Ill Patients in Skeletal Muscle and Brain
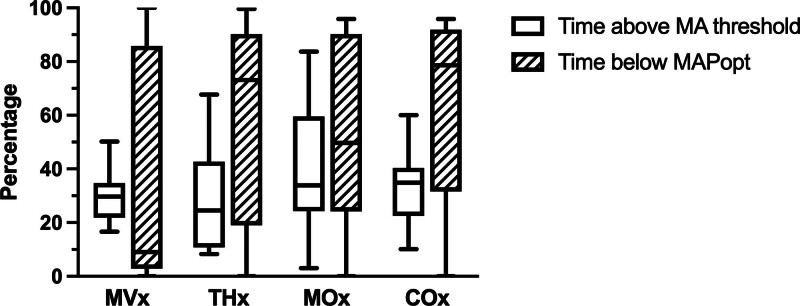
The correlation indices between each NIRS and MAP signal provide an evaluation of MA within skeletal muscle and brain. The median (IQR) value of each index is computed as follows: MVx: –0.02 (–0.06 to 0.09), THx: –0.02 (–0.11 to 0.20), MOx: 0.12 (0.02–0.28), and COx: 0.11 (0.05–0.21). These correlation indices also exhibit notable fluctuations within the same patient recording, with coefficients of variation of 1445% for MVx, 617% for THx, 1017% for MOx, and 418% for COx. To evaluate the extent of autoregulation impairment in patients, the percentage of time during which the correlation index of each signal exceeds the threshold of 0.3 is calculated. These values are reported as median (IQR): MVx: 29.7% (22.3–33.6%), THx: 24.5% (12.9–41.0%), MOx: 33.9% (24.9–56.0%), and COx: 34.9% (24.0–40.0%), as shown in Figure 1. Furthermore, to assess the magnitude of autoregulation impairment, the dose for which each signal remains above the 0.3 threshold per hour is computed, with values detailed in Supplementary Table 1 (http://links.lww.com/CCX/B353).
Figure 1.
The percentage of time that each microvascular autoregulation (MA) correlation index exceeds the impairment threshold of 0.3 and the percentage of time that mean arterial pressure (MAP) is below the calculated optimal MAP (MAPopt). Plots represent median, interquartile range, and minimum–maximum for the cohort. COx = brain tissue saturation index, MOx = muscle tissue saturation index, MVx = muscle total hemoglobin, THx = brain total hemoglobin.
Derived MAPopt From Skeletal Muscle and Cerebral NIRS Signals
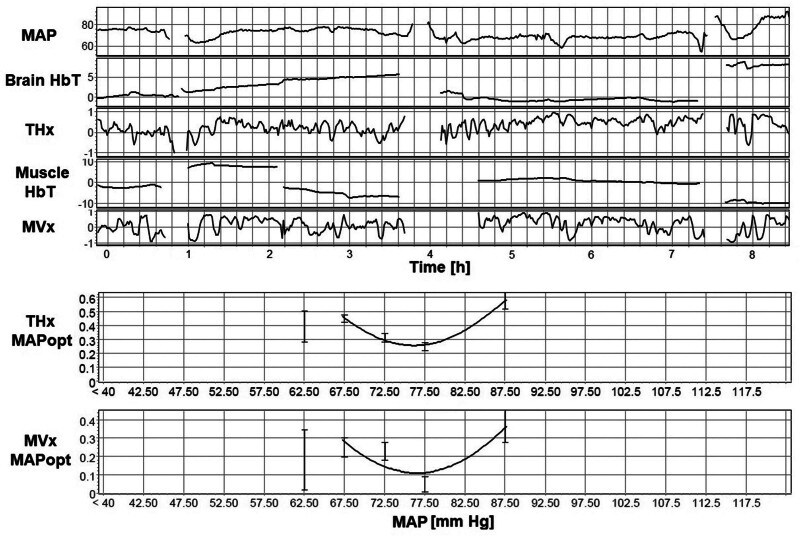
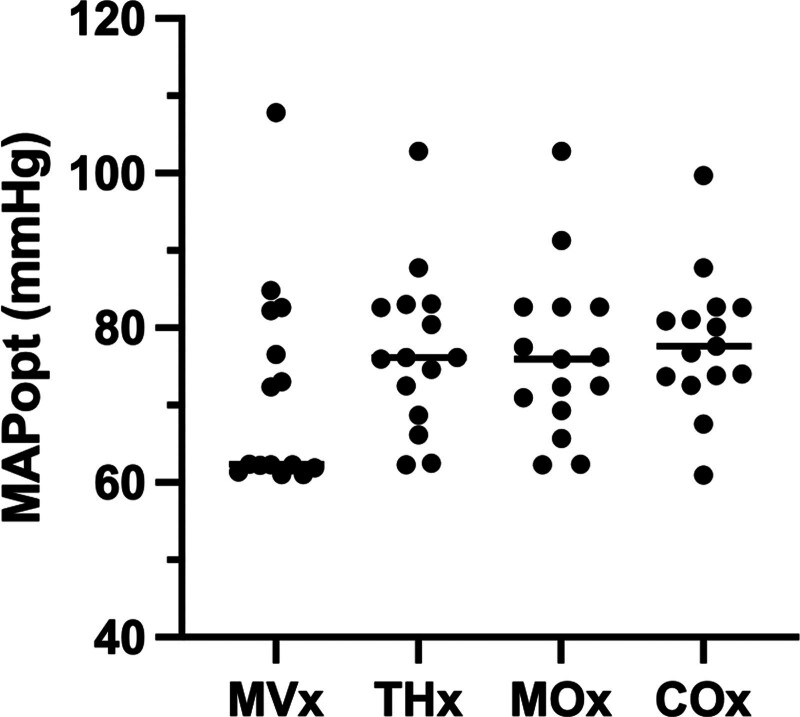
The MAPopt values were successfully determined for each correlation coefficient across all patients. Twenty-four of 60 signals required adjustment to default ICM+ settings, as described in Supplementary Table 1 (http://links.lww.com/CCX/B353). Figure 2 serves as an illustrative example of ICM+ output, showcasing the process of calculating MAPopt. This figure displays the MAP, THx, THx, MVx, and MVx signals recorded over an approximately 8-hour session. Areas with no data correspond to segments where motion artifacts were removed. The figure also demonstrates the mean values of THx and MVx plotted in 5 mm Hg bins of MAP, fitted by a U-shaped curve that identifies the MAPopt at its nadir. As shown in Figure 3, the calculated MAPopt values for the cohort are presented as median (IQR): MVx–MAPopt 62.35 mm Hg (62.07–79.40 mm Hg); THx–MAPopt 76.14 mm Hg (70.61–82.86 mm Hg); MOx–MAPopt 75.98 mm Hg (70.15–82.70 mm Hg); and COx–MAPopt 77.65 mm Hg (73.76–81.87 mm Hg). MAPopt values for each correlation index were above 65 mm Hg for seven patients (47%), 13 patients (87%), 12 patients (80%), and 14 patients (93%) for MVx, THx, MOx, and COx, respectively. Among the 60 MAPopt values analyzed in this study—four values for each MA index per patient—30 signals (50.0%) exhibited a U-shaped curve in their plot of MA index vs. MAP. Sixteen signals (26.7%) demonstrated a descending curve along the MAP axis, and 14 signals (23.3%) displayed an ascending curve. We also explored various algorithm configurations, involving window lengths of 1, 2, 4, and 8 hours, as well as bin widths of 3, 4, and 5 mm Hg. The median MAPopt, IQR, and yield percentage for each configuration are provided in Supplementary Table 1 (http://links.lww.com/CCX/B353); overall, there was low variability between each of these configurations with only modest differences in derived MAPopt. The proportion of time during which blood pressure remained below the determined MAPopt is also assessed, represented as median (IQR): 9.0% (3.0–77.5%) for MVx, 73.1% (25.0–87.0%) for THx, 49.7% (27.0–88.4%) for MOx, and 78.6% (46.7–89.5%) for COx, as shown in Figure 1.
Figure 2.
Derivation of optimal mean arterial pressure (MAPopt) from a representative time series from a single patient. Near-infrared spectroscopy signals from brain total hemoglobin (HbT) (THx) and muscle HbT (MVx), with the removal of motion artifacts and 10-s moving average filter, are used to derive a correlation index with arterial blood pressure denoted as THx and MVx, respectively. The error bar charts of THx and MVx vs. mean arterial pressure (MAP) bins are used to fit a U-shaped curve that calculates the nadir as MAPopt; this is the MAP with best preservation of microvascular autoregulation. In this particular patient, the fitted MAPopt curves derived from the brain and muscle exhibit a high degree of similarity, despite differences in the correlation indices for each MAP bin between the two organs.
Figure 3.
Optimal mean arterial pressure (MAPopt) values of the cohort derived from each of the four microvascular autoregulation (MA) indices. MA indices reflect the correlation between arterial blood pressure and muscle total hemoglobin (MVx), brain total hemoglobin (THx), muscle tissue saturation index (MOx), and brain tissue saturation index (COx). Line represents the median value.
Correlation and Comparison of MAPopt Values Derived From Skeletal Muscle and Brain NIRS
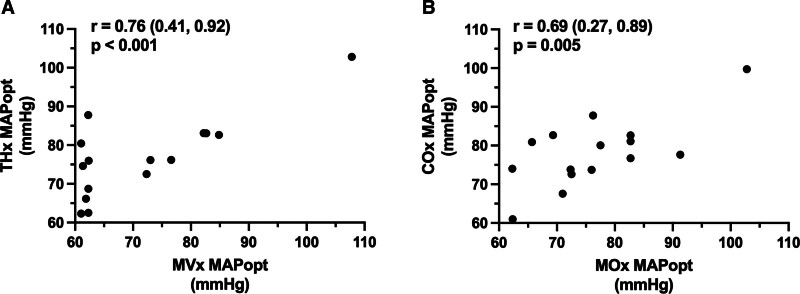
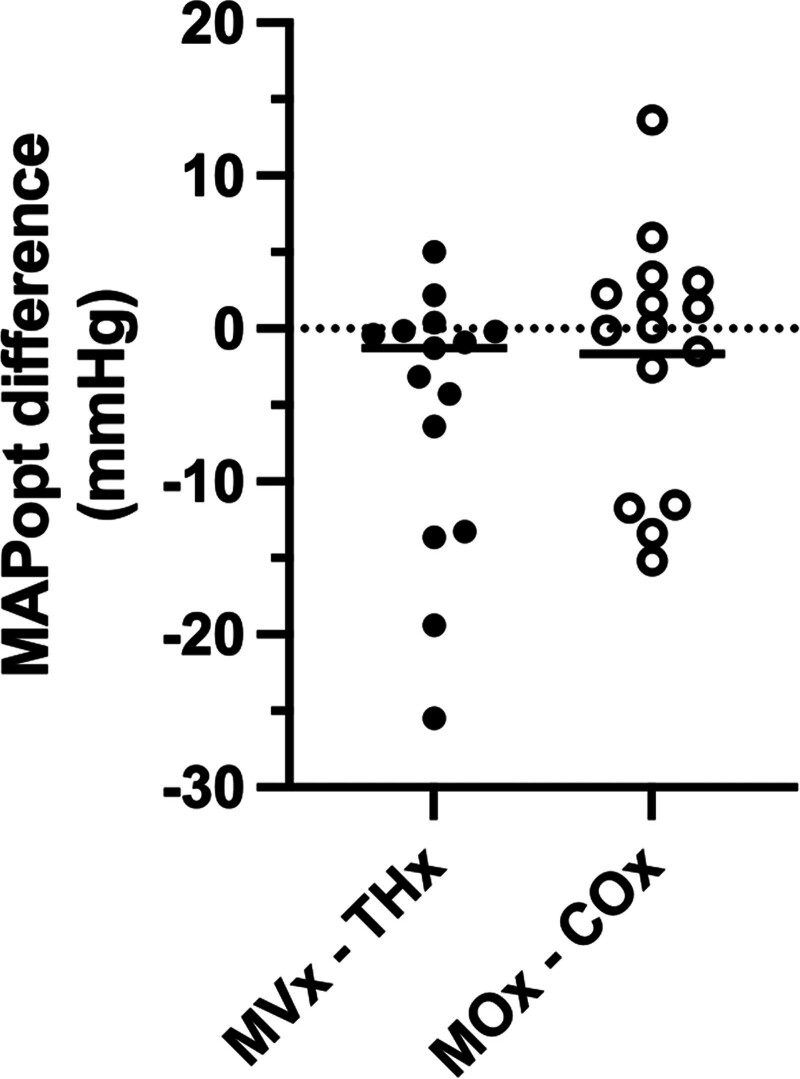
The correlation between skeletal muscle- and brain-derived MAPopt is shown in Figure 4. Pearson correlation coefficient between MVx–MAPopt and THx–MAPopt is r = 0.76 (p < 0.001; CI, 0.41–0.92), and between MOx–MAPopt and COx–MAPopt is r = 0.69 (p = 0.005; CI, 0.27–0.89), suggesting a moderate correlation between MAPopt values derived from muscle and THx and TSI signals. However, the difference between the calculated HbT–MAPopt values and the TSI-MAPopt values of the muscle and brain shows some notable differences. As shown in Figure 5, these calculations yielded a median (IQR) difference of –1.27 mm Hg (–9.85 to –0.18 mm Hg) and 0.05 mm Hg (–7.05 to 2.68 mm Hg), respectively. The correlation between HbT- and TSI-derived MAPopt values for the same patient was also calculated: MVx–MOx correlation is r = 0.58 (p = 0.022; CI, 0.10–0.84), and THx–COx correlation is r = 0.65 (p = 0.009; CI, 0.21–0.87).
Figure 4.
Correlation between optimal mean arterial pressure (MAPopt) values obtained from muscle and brain near-infrared spectroscopy (NIRS) for each patient in the cohort, derived from correlation indices from NIRS total hemoglobin (muscle total hemoglobin [MVx], brain total hemoglobin [THx]) and tissue saturation index (muscle tissue saturation index [MOx], brain tissue saturation index [COx]). A, Correlation between MVx–MAPopt and THx–MAPopt. B, Correlation between MOx–MAPopt and COx–MAPopt. Values represent Pearson correlation coefficient (95% CI).
Figure 5.
The difference between optimal mean arterial pressure (MAPopt) values of muscle and brain using near-infrared spectroscopy signals for total hemoglobin (muscle total hemoglobin [MVx]– brain total hemoglobin [THx]) and tissue saturation index (muscle tissue saturation index [MOx]–brain tissue saturation index [COx]) for each patient in the cohort. Across the cohort, MAPopt in skeletal muscle can higher, lower, or nearly identical to the MAPopt in the brain. Line represents the median value.
DISCUSSION
In this observational study, we used hr-NIRS to simultaneously monitor microvascular perfusion in skeletal muscle (brachioradialis) and brain (frontal cortex) in ICU patients requiring vasoactive medication on the first day of ICU admission. We determined the correlation between ABP and NIRS (HbT and TSI) at both anatomical sites and derived the optimal MAP (MAPopt) that minimizes this correlation coefficient—which is proposed to be the target MAP that best preserves MA. To the best of our knowledge, we have shown for the first time in an ICU setting the presence of MA in skeletal muscle, MAP-dependent variation in these MA indices, and the derivation of MAPopt for skeletal muscle. This study is also noteworthy for demonstrating in a general ICU population that: 1) patients experience a significant burden of microvascular dysfunction in both skeletal muscle and brain, as quantified by the proportion of time above the threshold of MA (0.3) on the first day of ICU admission and 2) MAPopt in skeletal muscle and brain can differ dramatically within the same patient and are not uniform between patients in the cohort.
The heterogeneity of MAPopt values in this cohort suggests an opportunity for personalized hemodynamic management based on microvascular status in patients with circulatory shock (17, 18), as has been proposed with CPPopt in traumatic brain injury (7, 29, 32). As shown in Figure 3, the MAPopt targets from both skeletal muscle and brain are commonly above the universal threshold of 65 mm Hg that is prescribed for critically ill patients. Asfar et al (33) did not show a benefit to targeting a MAP of 80 vs. 65 mm Hg in septic patients, but this was applied uniformly across the entire study population. The ANDROMEDA investigators have incorporated a “vasopressor test” of raising MAP with norepinephrine using a target of 80–85 mm Hg after volume resuscitation in patients with persistently abnormal CRT (4, 34). Interestingly, 12 of 15 patients in our current study had normal CRT of less than 3 seconds, despite many remaining below their MAPopt target. Huang et al (35) found that sublingual microcirculation was abnormal (microcirculatory flow index < 2.6) in 34 of 228 patients (15%) with preserved CRT. Therefore, future research should evaluate whether using advanced physiologic metrics such as MAPopt may confer additional benefits for selected patients above clinical markers of perfusion and how they can be incorporated into contemporary resuscitation protocols.
Our results align with previous literature, highlighting the prevalence of microvascular dysfunction (36, 37) and impaired cerebral MA (38, 39) in a general ICU population. While static (Sto2) and dynamic (vascular occlusion test) peripheral NIRS-derived indices correlate with adverse ICU outcomes in sepsis (40), integrating Sto2 into a treatment protocol did not improve survival, and resulted in more days of mechanical ventilation and RBC transfusion (41). Recently, Bruno et al (42) randomized patients with shock to provide clinicians with an assessment of sublingual microcirculation, but this did not confer a survival benefit despite frequent changes to management; this study was criticized for not connecting these sublingual measurements with a specified intervention (43). In this regard, skeletal muscle MAPopt presented in our study may overcome some of these limitations, as this will also define a specific treatment—titration of vasoactive medications to achieve the target MAPopt.
The comparison between MAPopt in skeletal muscle and brain is a novel and important finding from our study. Our data shows that although a correlation in MAPopt values exists between muscle and brain (Fig. 4), the actual MAPopt targets for skeletal muscle can be either above, below, or the same as for the brain and that these targets can differ depending on whether they are based on HbT- or TSI-derived indices of MA (Fig. 5). Our findings may suggest that hemodynamic resuscitation targets require not only patient-specific adjustments but also organ-specific considerations. Although MAP is a primary determinant of cerebral blood flow, MAP itself is determined by systemic vascular resistance in skeletal muscle. Furthermore, skeletal muscle blood flow control is under a high degree of sympathetic tone (14) and a primary site of action for vasoactive medications, whereas cerebrovascular networks exhibit predominantly myogenic control (44, 45) and MA in the brain appears relatively insensitive to changes in vasopressor dosage (46). Furthermore, the role of astrocytes and pericytes in microvascular flow regulation is well-described in the brain (47, 48), but corollary cell types and mechanisms in skeletal muscle are either absent or noncontributory. Prior studies have examined the discrepancies between sublingual microcirculation and intestinal perfusion (49, 50), as well as the agreement between peripheral NIRS and hepatosplanchnic blood flow (51), but a paucity of literature has examined the interactions between peripheral and cerebral perfusion using noninvasive monitoring techniques.
These organ-specific hemodynamic discrepancies raise interesting considerations regarding the pathophysiology of shock states the potential applications of MAPopt for resuscitation management. For example, redistribution of blood flow away from skeletal muscle is a protective mechanism to preserve vital organs (44), and CRT is a peripheral perfusion metric that is commonly used in the early phase of resuscitation. However, when discrepancies exist between resuscitation targets of various organs, as between skeletal muscle and brain MAPopt in this study, the priority and timing of how they are treated remains an unanswered question. Muscle MAPopt—as a marker of general perfusion—may be the initial resuscitation priority, and then brain MAPopt may require further optimization after general perfusion is achieved; alternatively, brain MAPopt might be a primary resuscitation target from the onset of shock. Furthermore, the impact of raising blood pressure with vasopressors to achieve MAPopt in one organ may have deleterious effects for other organs. The relatively short monitoring period in our study (hr) does not allow us to determine how MAPopt evolves throughout ICU admission (d), as has been shown with cerebral monitoring (9). This extended monitoring may determine how impaired MA and deviation from MAPopt relates to ICU outcomes such as duration of vasopressors, resolution of organ failure, and neurocognitive dysfunction (e.g., delirium), and inform how MAPopt targets can be incorporated into future resuscitation protocols.
This study is exploratory and has both technical and experimental limitations. Our use of research-grade hr-NIRS equipment enabled access to raw optical data at high temporal resolution (10 Hz) but was vulnerable to motion artifact; there is also no short-distance channel to remove superficial signals, which can affect cerebral measurements. Furthermore, our use of a fixed-window MAPopt derivation was suitable for short monitoring sessions, but newer algorithms are currently being evaluated in the literature, both within existing software (e.g., optimal flex window methodology [23]) or designed de novo by research groups (38); newer methodology is still required to derive MAPopt from continuous physiologic data in real time at the bedside. The choice of HbT- or TSI-derived indices of MA to best determine MAPopt is not established; a comprehensive study in a swine model of cardiac arrest compared many indices (52), and human studies in traumatic brain injury show that these indices behave similarly (53). The small sample size of this cohort is meant to demonstrate the ability to calculate MAPopt in skeletal muscle and is not intended to make general claims about specific target values. Larger studies may also help elucidate the contribution of chronic comorbidities (e.g., diabetes, peripheral arterial disease) to the observed microvascular dysfunction. Last, it should be noted that, unlike for cerebral monitoring, impaired MA in skeletal muscle has not yet been associated with adverse ICU outcomes, nor is the threshold for abnormal MA of 0.3 identified in skeletal muscle. This will be a focus for our future research.
CONCLUSIONS
Measurement of microvascular autoregulation (MA) in skeletal muscle is feasible using NIRS in the ICU and can enable the calculation of MAPopt, which may serve as a novel resuscitation target. MAPopt values are not uniform for all patients in the ICU, and are frequently above the universal 65 mm Hg threshold. Furthermore, MAPopt values derived from skeletal muscle can be significantly different from those derived in the brain. Future studies should explore the association between impaired skeletal muscle MA, ICU outcomes, and organ-specific differences in MA and MAPopt thresholds.
ACKNOWLEDGMENTS
We thank Dr. Rahul Kher for his preliminary assistance with the project.
Supplementary Material
Footnotes
Microvasclar Monitoring in Circulatory Shock and Sepsis (MiMICSS) Investigators are available at: http://links.lww.com/CCX/B352.
Dr. Mendelson was involved in study conceptualization. Mr. Mirsajadi, Mr. Erickson, Ms. Alias, Ms. Wilson, Mr. Herath, and Dr. Mendelson were involved in data acquisition. Mr. Mirsajadi, Dr. Froese, Mr. Singh Sainbhi, Mr. Herath, and Mr. Majumdar were involved in data analysis. Dr. Mendelson, Dr. Zeiler, Dr. Froese, Dr. Gomez, and Dr. Zarychanski were involved in investigation. Dr. Mendelson was involved in study funding. Mr. Mirsajadi and Dr. Mendelson were involved in article writing (first draft). All authors were involved in article edit and final approval.
Presented, in part, as a poster at the Critical Care Canada Forum, Toronto, ON, Canada, November 2023.
This work is supported by an operating grant from the Department of Internal Medicine at the University of Manitoba (to Dr. Mendelson).
Dr. Mendelson receives research support from the Manitoba Medical Services Foundation Dr. F. W. DuVal and John Henson Clinical Research Professorship. Drs. Mendelson and Zeiler have funding, that is, unrelated to this current project from the Health Sciences Centre Foundation (Winnipeg), the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation, and the Research Manitoba. Dr. Gomez is supported by the CIHR Fellowship program. Mr. Singh Sainbhi is supported by the University of Manitoba Graduate Fellowship in Biomedical Engineering—Doctoral Stream. Dr. Froese is supported through the Natural Sciences and Engineering Research Council of Canada (NSERC) Post-Doctoral Fellowship program. Dr. Zeiler is supported through the University of Manitoba Endowed Manitoba Public Insurance Chair in Neuroscience and the NSERC (DGECR-2022-00260, RGPIN-2022-03621, ALLRP-578524-22, ALLRP-576386-22, ALLRP-586244-23, I2IPJ 586104–23). Dr. Zarychanski receives research support from the Lyonel G. Israels Endowed Research Chair in Hematology, University of Manitoba; he has funding, that is, unrelated to the current project from the CIHR. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Amirali Mirsajadi, Email: mirsajaa@myumanitoba.ca.
Dustin Erickson, Email: ericks26@myumanitoba.ca.
Soumya Alias, Email: aliass3@myumanitoba.ca.
Logan Froese, Email: froesel3@myumanitoba.ca.
Amanjyot Singh Sainbhi, Email: amanjyot.s.sainbhi@gmail.com.
Alwyn Gomez, Email: alwyn.gomez@umanitoba.ca.
Raju Majumdar, Email: raju.majumdar@umanitoba.ca.
Isuru Herath, Email: herathi1@myumanitoba.ca.
Maggie Wilson, Email: maggie.wilson@umanitoba.ca.
Frederick A. Zeiler, Email: frederick.zeiler@umanitoba.ca.
Collaborators: Asher Mendelson, Frederick Zeiler, Ryan Zarychanski, Sylvain Lother, Gloria Vazquez Grande, Olawale Ayilara, J Gordon Boyd, David Maslove, and Stephanie Sibley
REFERENCES
- 1.Cecconi M, De Backer D, Antonelli M, et al. : Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014; 40:1795–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kattan E, Ibarra-Estrada M, Ospina-Tascón G, et al. : Perspectives on peripheral perfusion assessment. Curr Opin Crit Care 2023; 29:208–214 [DOI] [PubMed] [Google Scholar]
- 3.Dubin A, Henriquez E, Hernández G: Monitoring peripheral perfusion and microcirculation. Curr Opin Crit Care 2018; 24:173–180 [DOI] [PubMed] [Google Scholar]
- 4.Hernández G, Ospina-Tascón GA, Damiani LP, et al. ; The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN): Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK randomized clinical trial. JAMA 2019; 321:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ince C: Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015; 19:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeiler FA, Ercole A, Czosnyka M, et al. : Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: A narrative review of advances in neurocritical care. Br J Anaesth 2020; 124:440–453 [DOI] [PubMed] [Google Scholar]
- 7.Beqiri E, Smielewski P, Robba C, et al. : Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: The COGiTATE phase II study protocol. BMJ Open 2019; 9:e030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aries MJH, Wesselink R, Elting JWJ, et al. : Enhanced visualization of optimal cerebral perfusion pressure over time to support clinical decision making. Crit Care Med 2016; 44:e996–e999 [DOI] [PubMed] [Google Scholar]
- 9.Aries MJH, Czosnyka M, Budohoski KP, et al. : Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012; 40:2456–2463 [DOI] [PubMed] [Google Scholar]
- 10.Donnelly J, Czosnyka M, Adams H, et al. : Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: A retrospective, single-center analysis. Neurosurgery 2019; 85:E75–E82 [DOI] [PubMed] [Google Scholar]
- 11.Zeiler FA, Ercole A, Beqiri E, et al. ; CENTER-TBI High Resolution ICU (HR ICU) Sub-Study Participants and Investigators: Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult traumatic brain injury: A CENTER-TBI study. J Neurotrauma 2020; 37:1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svedung Wettervik T, Howells T, Enblad P, et al. : Temporal neurophysiological dynamics in traumatic brain injury: Role of pressure reactivity and optimal cerebral perfusion pressure for predicting outcome. J Neurotrauma 2019; 36:1818–1827 [DOI] [PubMed] [Google Scholar]
- 13.Sorrentino E, Diedler J, Kasprowicz M, et al. : Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 2012; 16:258–266 [DOI] [PubMed] [Google Scholar]
- 14.Bagher P, Segal SS: Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol (Oxf) 2011; 202:271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murrant CL, Sarelius IH: Local control of blood flow during active hyperaemia: What kinds of integration are important? J Physiol 2015; 593:4699–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner MJ, Casey DP: Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol Rev 2015; 95:549–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Backer D, Cecconi M, Chew MS, et al. : A plea for personalization of the hemodynamic management of septic shock. Crit Care 2022; 26:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato R, Pinsky MR: Personalizing blood pressure management in septic shock. Ann Intens Care 2015; 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JK, Kibler KK, Benni PB, et al. : Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 2009; 40:1820–1826 [DOI] [PubMed] [Google Scholar]
- 20.Zweifel C, Castellani G, Czosnyka M, et al. : Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke 2010; 41:1963–1968 [DOI] [PubMed] [Google Scholar]
- 21.Froese L, Gomez A, Sainbhi AS, et al. : Continuous determination of the optimal bispectral index value based on cerebrovascular reactivity in moderate/severe traumatic brain injury: A retrospective observational cohort study of a novel individualized sedation target. Crit Care Explor 2022; 4:e0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner LA, Czosnyka M, Piechnik SK, et al. : Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002; 30:733–738 [DOI] [PubMed] [Google Scholar]
- 23.Beqiri E, Ercole A, Aries MJ, et al. : Optimal cerebral perfusion pressure assessed with a multi-window weighted approach adapted for prospective use: A validation study. Acta Neurochir Suppl 2021; 131:181–185 [DOI] [PubMed] [Google Scholar]
- 24.Crippa IA, Subirà C, Vincent J-L, et al. : Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care 2018; 22:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sainbhi AS, Froese L, Gomez A, et al. : Continuous time-domain cerebrovascular reactivity metrics and discriminate capacity for the upper and lower limits of autoregulation: A scoping review of the animal literature. Neurotrauma Rep 2021; 2:639–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein KY, Froese L, Sekhon M, et al. : Intracranial pressure-derived cerebrovascular reactivity indices and their critical thresholds: A Canadian high resolution-traumatic brain injury validation study. J Neurotrauma 2023; 41:910–923 [DOI] [PubMed] [Google Scholar]
- 27.Riemann L, Beqiri E, Younsi A, et al. : Predictive and discriminative power of pressure reactivity indices in traumatic brain injury. Neurosurgery 2020; 87:655–663 [DOI] [PubMed] [Google Scholar]
- 28.Beqiri E, Zeiler FA, Ercole A, et al. ; CENTER-TBI HR ICU participants and investigators: The lower limit of reactivity as a potential individualised cerebral perfusion pressure target in traumatic brain injury: A CENTER-TBI high-resolution sub-study analysis. Crit Care 2023; 27:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiler FA, Ercole A, Cabeleira M, et al. ; CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators: Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult traumatic brain injury: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 2019; 36:1505–1517 [DOI] [PubMed] [Google Scholar]
- 30.Needham E, McFadyen C, Newcombe V, et al. : Cerebral perfusion pressure targets individualized to pressure-reactivity index in moderate to severe traumatic brain injury: A systematic review. J Neurotrauma 2017; 34:963–970 [DOI] [PubMed] [Google Scholar]
- 31.Stein KY, Froese L, Gomez A, et al. : Time spent above optimal cerebral perfusion pressure is not associated with failure to improve in outcome in traumatic brain injury. Intensive Care Med Exp 2023; 11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly J, Czosnyka M, Adams H, et al. : Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med 2017; 45:1464–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asfar P, Meziani F, Hamel J-F, et al. ; SEPSISPAM Investigators: High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014; 370:1583–1593 [DOI] [PubMed] [Google Scholar]
- 34.Kattan E, Bakker J, Estenssoro E, et al. : Hemodynamic phenotype-based, capillary refill time-targeted resuscitation in early septic shock: The ANDROMEDA-SHOCK-2 Randomized Clinical Trial study protocol. Rev Bras Ter Intensiva 2022; 34 :96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang W, Xiang H, Hu C, et al. : Association of sublingual microcirculation parameters and capillary refill time in the early phase of ICU admission*. Crit Care Med 2023; 51:913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Backer D, Donadello K, Sakr Y, et al. : Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit Care Med 2013; 41:791–799 [DOI] [PubMed] [Google Scholar]
- 37.Donati A, Damiani E, Domizi R, et al. : Near-infrared spectroscopy for assessing tissue oxygenation and microvascular reactivity in critically ill patients: A prospective observational study. Critical Care 2016; 20:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KF, Wood MD, Maslove DM, et al. : Dysfunctional cerebral autoregulation is associated with delirium in critically ill adults. J Cereb Blood Flow Metab 2019; 39:2512–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan JM, Shore A, Lee KFH, et al. : Cerebral autoregulation-based mean arterial pressure targets and delirium in critically ill adults without brain injury: A retrospective cohort study. Can J Anaesth 2024; 71:107–117 [DOI] [PubMed] [Google Scholar]
- 40.Neto AS, Pereira VGM, Manetta JA, et al. : Association between static and dynamic thenar near-infrared spectroscopy and mortality in patients with sepsis. J Trauma Acute Care Surg 2014; 76:226–233 [DOI] [PubMed] [Google Scholar]
- 41.Nardi O, Zavala E, Martin C, et al. : Targeting skeletal muscle tissue oxygenation (StO2) in adults with severe sepsis and septic shock: A randomised controlled trial (OTO-StS Study). BMJ Open 2018; 8:e017581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruno RR, Wollborn J, Fengler K, et al. : Direct assessment of microcirculation in shock: A randomized-controlled multicenter study. Intensive Care Med 2023; 49:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilty MP, Duranteau J, Montomoli J, et al. : A microcirculation-guided trial doomed to fail. Intensive Care Med 2023; 49:1557–1558 [DOI] [PubMed] [Google Scholar]
- 44.Lima A, Bakker J: Clinical monitoring of peripheral perfusion: There is more to learn. Crit Care 2014; 18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willie CK, Tzeng Y-C, Fisher JA, et al. : Integrative regulation of human brain blood flow. J Physiol 2014; 592:841–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froese L, Gomez A, Sainbhi AS, et al. : Temporal relationship between vasopressor and sedative administration and cerebrovascular response in traumatic brain injury: A time-series analysis. Intensive Care Med Exp 2023; 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacVicar BA, Newman EA: Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 2015; 7:a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann DA, Coelho-Santos V, Shih AY: Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol 2022; 84:331–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boerma EC, van der Voort PHJ, Spronk PE, et al. : Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis*. Crit Care Med 2007; 35:1055–1060 [DOI] [PubMed] [Google Scholar]
- 50.Edul VSK, Ince C, Navarro N, et al. : Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann Intensive Care 2014; 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez G, Regueira T, Bruhn A, et al. : Relationship of systemic, hepatosplanchnic, and microcirculatory perfusion parameters with 6-hour lactate clearance in hyperdynamic septic shock patients: An acute, clinical-physiological, pilot study. Ann Intensive Care 2012; 2:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govindan RB, Brady KM, Massaro AN, et al. : Comparison of frequency- and time-domain autoregulation and vasoreactivity indices in a piglet model of hypoxia-ischemia and hypothermia. Dev Neurosci 2018; 40:547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeiler FA, Donnelly J, Menon DK, et al. : Continuous autoregulatory indices derived from multi-modal monitoring: Each one is not like the other. J Neurotrauma 2017; 34:3070–3080 [DOI] [PubMed] [Google Scholar]