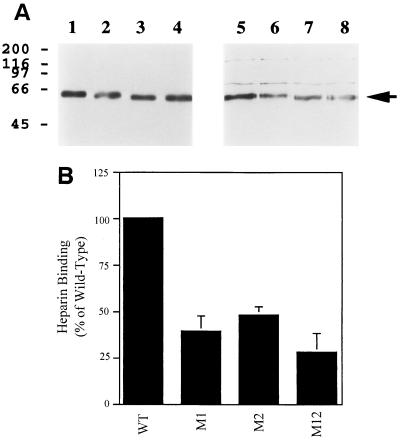
FIG. 4.
Analysis of heparin-binding by mutated derivatives of HHV-7 gp65. Mutated derivatives of HHV-7 gp65 were constructed, and the proteins were then expressed in insect cells, with a C-terminal (His6) epitope tag. Heparin-binding by the proteins was then assessed (see Fig. 3 for methods). (A) The results of one representative heparin-binding experiment are shown. Lanes are as follows: 1 to 4, input protein (directly loaded onto the gel and then probed by immunoblotting with the anti-His6 monoclonal antibody); 5 to 8, protein bound to heparin-acrylate beads and then analyzed by immunoblotting with the anti-His6 monoclonal antibody. Wild-type gp65-(His6) was analyzed in lanes 1 and 5, while mutant derivatives of gp65-(His6) were analyzed in the other lanes (lanes 2 and 6, M1; lanes 3 and 7, M2; lanes 4 and 8, M12). (B) Results from four independent heparin-binding experiments (including the one shown in panel A) were densitometrically quantitated and normalized in terms of input protein loaded (as detected by the anti-His6 immunoblot assay). The amount of the mutant proteins which bound to heparin-agarose beads was then expressed as a percentage of heparin-binding by wild-type gp65-(His6), and mean binding values were plotted in the figure; bars represent the standard error of the means.