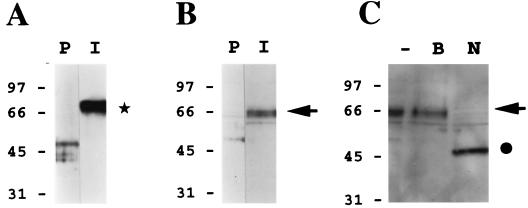
FIG. 6.
HHV-7 gp65 is present within highly purified HHV-7 virion preparations. A murine gp65-specific polyclonal antiserum (see Fig. 5) was used to perform immunoblot analyses of sucrose density gradient-purified HHV-7 virion preparations. (A) The immune antiserum (I) or its preimmune counterpart (P) was reacted with a purified gp65-Ig fusion protein (this fusion protein contained amino acid residues 23 to 325 of gp65, fused in frame to the Fc portion of human immunoglobulin G, contained in the signal pIg-Tail vector; Novagen, Inc.). As shown by the asterisk, only the immune antiserum reacted with the gp65-Ig protein (ca. 70 kDa). (B) The immune antiserum (I) or its preimmune counterpart (P) was reacted with a lysate of sucrose density gradient-purified HHV-7 virions. The arrow denotes the specific reactivity of the immune antiserum with a protein doublet of roughly 65 kDa. (C) The HHV-7 virion lysate was subjected to extensive digestion with N-glycanase (PNGase F) prior to immunoblot analysis with the immune antiserum (N). In control experiments, the lysate was incubated in reaction buffer alone (i.e., no PNGase was added) under the same conditions (B), or the lysate was analyzed directly, without prior treatment in vitro (−). The arrow denotes the position of HHV-7 gp65, while the filled circle shows the size of the product of PNGase digestion of this molecule (ca. 50 kDa).