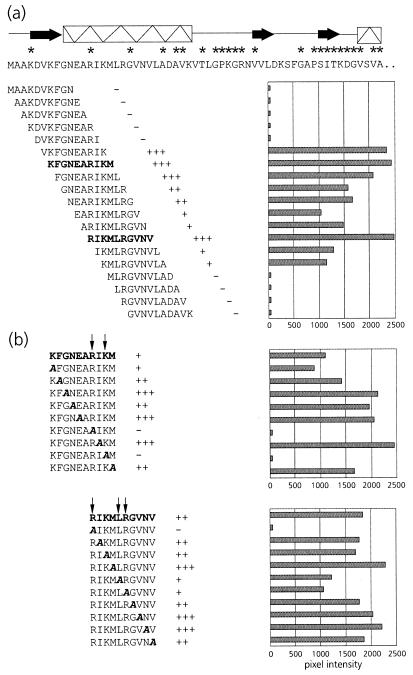
FIG. 2.
(a) Schematic representation of PLRV-binding activities of synthetic decameric peptides corresponding to amino acid residues 1 to 57 of the N-terminal region of the equatorial domain of MpB GroEL. The results of the first 19 peptides are shown; no PLRV binding to any of the subsequent peptides in this region was detected. Secondary structural elements are indicated by thick arrows (β-strands) and boxed sine waves (α-helices). Conserved sequences in GroEL/Hsp60 sequences are indicated by asterisks (14). (b) Alanine scanning of the two decameric peptides with the strongest binding capacities (boldfaced). The affinity of PLRV for the peptides has been quantified using Molecular Analyst software (histograms) and is interpreted as follows: +++, high affinity; ++, intermediate affinity; +, low affinity; −, no PLRV binding detected. Arrows indicate residues critical for binding PLRV.