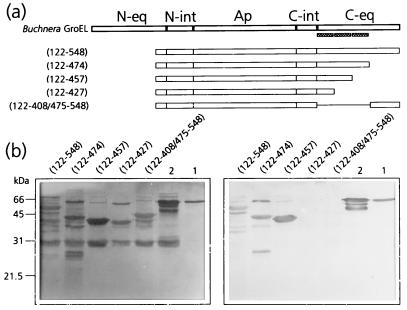
FIG. 4.
Mapping of the C-terminal PLRV-binding site by virus overlay assays of deletion derivatives of MpB GroEL. (a) Schematic representation of MpB GroEL deletion mutants. The numbers in parentheses correspond to the positions of amino acid residues of MpB GroEL (17) and mark the borders of the deletion mutants. N-eq, N-terminal region of the equatorial domain; N-int, N-terminal region of the intermediate domain; Ap, apical domain; C-int, C-terminal region of the intermediate domain; C-eq, C-terminal region of the equatorial domain. Secondary structural elements are indicated by boxed sine waves (α-helices). (b) Virus overlay assay (right) showing that the putative α-helix between residues 427 and 457 is part of the PLRV-binding site. Lanes 1, wild-type MpB GroEL isolated from M. persicae; lanes 2, recombinant MpB GroEL. All other lanes contain the indicated deletion mutants of MpB GroEL as depicted in panel a. (Left) Amido black-stained blot.