This secondary analysis of a randomized clinical trial examines the occurrence of target vessel failure and the role of diabetes in the clinical outcomes of patients undergoing complex percutaneous coronary intervention.
Key Points
Questions
Is the benefit of intravascular imaging guidance during complex percutaneous coronary intervention (PCI) different in the presence of diabetes?
Findings
In this prespecified secondary analysis of a subgroup of 1639 participants in the RENOVATE-COMPLEX-PCI randomized clinical trial, intravascular imaging guidance reduced the risk of target vessel failure compared with angiography guidance in patients without diabetes but not in patients with diabetes during complex PCI. Among patients with diabetes, only those with good glycemic control and who achieved stent optimization by intravascular imaging had a lower risk of future ischemic events.
Meaning
Findings of this study suggest that in patients with diabetes undergoing complex PCI, attention should be paid to stent optimization using intravascular imaging as well as glycemic control to improve outcomes.
Abstract
Importance
Data are limited regarding the effects of intravascular imaging guidance during complex percutaneous coronary intervention (PCI) in patients with diabetes.
Objective
To compare the clinical outcomes of intravascular imaging–guided vs angiography-guided complex PCI in patients with or without diabetes.
Design, Setting, and Participants
This prespecified secondary analysis of a subgroup of patients in RENOVATE-COMPLEX-PCI (Randomized Controlled Trial of Intravascular Imaging Guidance Versus Angiography-Guidance on Clinical Outcomes After Complex Percutaneous Coronary Intervention), an investigator-initiated, open-label multicenter trial, analyzed enrolled patients who underwent complex PCI at 20 sites in Korea from May 2018 through May 2021. Eligible patients were randomly assigned in a 2:1 ratio to undergo either the intravascular imaging–guided PCI or angiography-guided PCI. Data analyses were performed from June 2023 to April 2024.
Interventions
Percutaneous coronary intervention was performed either under the guidance of intravascular imaging or angiography alone.
Main Outcomes and Measures
The primary end point was target vessel failure (TVF), defined as a composite of cardiac death, target vessel–related myocardial infarction, or target vessel revascularization.
Results
Among the 1639 patients included in the analysis (mean [SD] age, 65.6 [10.2] years; 1300 males [79.3%]), 617 (37.6%) had diabetes. The incidence of TVF was significantly higher in patients with diabetes than patients without diabetes (hazard ratio [HR], 1.86; 95% CI, 1.33-2.60; P < .001). Among patients without diabetes, the intravascular imaging–guided PCI group had a significantly lower incidence of TVF compared with the angiography-guided PCI group (4.7% vs 12.2%; HR, 0.41 [95% CI, 0.25-0.67]; P < .001). Conversely, in patients with diabetes, the risk of TVF was not significantly different between the 2 groups (12.9% vs 12.3%; HR, 0.97 [95% CI, 0.60-1.57]; P = .90). There was a significant interaction between the use of intravascular imaging and diabetes for the risk of TVF (P for interaction = .02). Among patients with diabetes, only those with good glycemic control (hemoglobin A1c level ≤7.5%) and who achieved stent optimization by intravascular imaging showed a lower risk of future ischemic events (HR, 0.31; 95% CI, 0.12-0.82; P = .02).
Conclusions and Relevance
In this secondary analysis of a subgroup of patients in the RENOVATE-COMPLEX-PCI trial, intravascular imaging guidance reduced the risk of TVF compared with angiography guidance in patients without diabetes (but not in patients with diabetes) during complex PCI. In patients with diabetes undergoing complex PCI, attention should be paid to stent optimization using intravascular imaging and glycemic control to improve outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT03381872
Introduction
Diabetes is a common comorbidity in coronary artery disease (CAD) and is associated with a higher risk of adverse events after percutaneous coronary intervention (PCI), even in the current-generation drug-eluting stent (DES) era.1,2,3 The increased risk of ischemic events after PCI for patients with diabetes has been linked to platelet hyperactivity, impaired endothelial dysfunction, diffuse atherosclerotic burden with severe calcification, smaller vessel size, and uneven neointimal strut coverage following DES implantation.4,5,6,7 In this regard, several randomized clinical trials and meta-analyses showed that per-vessel treatment with coronary artery bypass graft (CABG) was superior to per-lesion treatment with PCI for minimizing the risk of ischemic events in patients with diabetes and complex CAD.8,9,10,11 Based on these findings, the current guidelines recommend CABG as the preferred method of revascularization for patients with diabetes, particularly those with complex CAD involving multiple coronary vessels.12,13
For complex anatomy, intravascular imaging, such as intravascular ultrasonography (IVUS) or optical coherence tomography (OCT), can offer valuable insights for stent optimization and has been associated with reduced risk of major adverse clinical events after PCI.14,15,16,17 In the RENOVATE-COMPLEX-PCI (Randomized Controlled Trial of Intravascular Imaging Guidance Vs Angiography-Guidance on Clinical Outcomes After Complex Percutaneous Coronary Intervention) trial, intravascular imaging–guided PCI was associated with a reduced risk of target vessel failure (TVF) compared with angiography-guided PCI for patients with complex coronary artery lesions.18 Given that patients with diabetes were at higher risk of late lumen loss and restenosis despite IVUS-guided stent optimization,19 the effectiveness of intravascular imaging guidance during complex PCI may differ according to the presence of diabetes. Therefore, the current study aimed to compare the clinical outcomes of intravascular imaging–guided vs angiography-guided complex PCI in patients with or without diabetes. In addition, we evaluated the implications of stent optimization by imaging and glycemic control for the clinical outcomes among patients with diabetes after complex PCI.
Methods
Study Design
We performed a prespecified secondary analysis of a subgroup of patients in RENOVATE-COMPLEX-PCI, an investigator-initiated, prospective, open-label multicenter randomized clinical trial conducted at 20 sites in Korea.18 The inclusion and exclusion criteria are provided in the eMethods in Supplement 1. In brief, patients who underwent PCI for complex coronary artery lesions were recruited. For the current study, we stratified trial participants according to their diabetes status and allocation groups (eFigure 1 in Supplement 1). All patients provided written informed consent before randomization. The Institutional Review Board at each participating center approved the study protocol (Supplement 2). We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Procedures
Eligible patients were randomly assigned in a 2:1 ratio to either the intravascular imaging–guided PCI or angiography-guided PCI. Randomization was performed by a web-based program.
Percutaneous coronary intervention was performed according to current clinical guidelines.12,13 The choice of intravascular imaging (IVUS or OCT) was at the operator’s discretion, with consideration of the complexity of coronary artery lesions and patient characteristics. While there were no restrictions on the timing of intravascular imaging, evaluations for optimization of the stented segment after PCI were obligatory. Standard protocols for intravascular imaging were followed during the procedure, and a commercially available IVUS (Opticross; Boston Scientific Corporation) or OCT (Dragonfly; Abbott Vascular) system was used.
Detailed protocols for image acquisition, optimization criteria of the stented segment, and medical treatment after PCI are provided in Supplement 2. Briefly, the optimal stent expansion thresholds were as follows: minimum stent area (MSA) greater than 5.5 mm2 on IVUS or greater than 4.5 mm2 on OCT for non–left main stenosis, residual diameter stenosis less than 10%, and MSA greater than 80% of average reference lumen area (coronary angiography). For left main lesions, the cutoff values for optimization were an absolute MSA greater than 7 mm2 for distal left main and greater than 8 mm2 for proximal left main.14 Major stent malapposition was defined as an acute malapposition with the distance between vessel wall and stent of 0.4 mm or longer, with longitudinal length over 1 mm. Major edge dissection was defined as dissection occurring within 5 mm from the edge of the stent, extending to the media layer with a dissection angle of at least 60° of the circumference of the vessel and/or 3 mm or more in length of the dissection flap. If any of these findings were detected, adjunctive postdilation or additional stent implantation was mandatory for optimizing stent placement. Adequate stent expansion without major malapposition or edge dissection was defined as optimized procedural results in imaging-guided PCI. All images, including those from angiography and intravascular imaging, were quantitatively analyzed in an independent core laboratory. A committee adjudicated clinical events without knowledge of group assignments.
End Points and Definitions
The primary end point was TVF, defined as a composite of cardiac death, target vessel–related myocardial infarction (MI), and clinically driven target vessel revascularization. Secondary end points were TVF without procedure-related MI, cardiac death or target vessel–related MI, all-cause death, cardiac death, target vessel MI with procedure-related MI, target vessel MI without procedure-related MI, any MI with procedure-related MI, any MI without procedure-related MI, non–target vessel–related MI, target lesion revascularization, target vessel revascularization, any revascularization (clinically driven), stent thrombosis, incidence of contrast-induced nephropathy, total amount of contrast use, total procedural time, and total medical cost.
Definitions of all secondary end points are presented in the eMethods in Supplement 1. Diabetes was defined as patients treated with oral hypoglycemic agents or insulin or patients with a glycosylated hemoglobin (HbA1c) level of 6.5% or greater. Among patients with diabetes, poor glycemic control was defined as an HbA1c level greater than 7.5%.20 The HbA1c level was collected at the index PCI date, 6 months after PCI, and 12 months after PCI.
Statistical Analysis
The statistical analysis plan for the RENOVATE-COMPLEX-PCI trial has been previously described.18 All analyses followed an intention-to-treat approach. Kaplan-Meier analyses were used to evaluate the cumulative incidence of end points, with significance assessed using the log-rank test. Treatment effects were estimated using Cox proportional hazards regression models, providing hazard ratios (HRs) and 95% CIs. The assumptions of the Cox proportional hazards regression models were assessed using the Schoenfeld residuals. Interaction assessment by diabetes and use of intravascular imaging for the risk of TVF was prespecified in the RENOVATE-COMPLEX-PCI trial. We further performed post hoc interaction assessment by glycemic control and stent optimization confirmed by intravascular imaging for the risk of TVF. Restricted cubic spline curves with 3 knots were used to identify the continuous effects of HbA1c on TVF.
Statistical analyses were performed from June 2023 to April 2024 using R for Windows, version 4.1.2 (R Project for Statistical Computing). Two-sided P < .05 indicated statistical significance.
Results
From May 2018 to May 2021, a total of 1639 patients with complex coronary artery lesions who underwent PCI (mean [SD] age, 65.6 [10.2] years; 1300 males [79.3%], 339 females [20.7%]) were recruited in the RENOVATE-COMPLEX-PCI. Among these patients, there were 617 (37.6%) with diabetes and 1022 (62.4%) without diabetes. Compared with patients without diabetes, those with diabetes were older; were less likely to be male; and had a higher proportion of conventional cardiovascular risk factors, including hypertension, dyslipidemia, chronic kidney insufficiency, history of PCI, history of stroke, and peripheral artery disease (Table 1 and eTable 1 in Supplement 1).
Table 1. Baseline Demographic Characteristics of Patients With Imaging-Guided or Angiography-Guided PCI by Diabetes Status.
| Characteristic | Patients with diabetes, No. (%) (n = 617) | Patients without diabetes, No. (%) (n = 1022) | ||||
|---|---|---|---|---|---|---|
| Imaging-guided PCI (n = 394) | Angiography-guided PCI (n = 223) | P value | Imaging-guided PCI (n = 698) | Angiography-guided PCI (n = 324) | P value | |
| Age, mean (SD), y | 66.9 (9.5) | 66.5 (9.1) | .67 | 64.5 (10.6) | 65.7 (10.5) | .09 |
| Sex | ||||||
| Female | 95 (24.1) | 50 (22.4) | .71 | 128 (18.3) | 66 (20.4) | .49 |
| Male | 299 (75.9) | 173 (77.6) | 570 (81.7) | 258 (79.6) | ||
| BMI, mean (SD) | 24.8 (3.6) | 25.0 (3.2) | .34 | 24.8 (3.3) | 24.8 (3.0) | .83 |
| Initial presentation | ||||||
| Stable IHD | 205 (52.0) | 118 (52.9) | .90 | 327 (46.8) | 157 (48.5) | .68 |
| Acute coronary syndrome | 189 (48.0) | 105 (47.1) | 371 (53.2) | 167 (51.5) | ||
| Unstable angina | 125 (66.1) | 69 (65.7) | 236 (63.6) | 104 (62.3) | ||
| AMI | 64 (33.9) | 36 (34.3) | 135 (36.4) | 63 (37.7) | ||
| Medical history | ||||||
| Hypertension | 294 (74.6) | 158 (70.9) | .36 | 388 (55.6) | 165 (50.9) | .19 |
| Dyslipidemia | 245 (62.2) | 137 (61.4) | .92 | 315 (45.1) | 143 (44.1) | .82 |
| Current smoking | 73 (18.5) | 37 (16.6) | .62 | 139 (19.9) | 58 (17.9) | .50 |
| Chronic kidney insufficiency | 98 (24.9) | 47 (21.1) | .33 | 105 (15.0) | 46 (14.2) | .80 |
| Previous PCI | 129 (32.7) | 64 (28.7) | .34 | 139 (19.9) | 63 (19.4) | .93 |
| Previous MI | 37 (9.4) | 21 (9.4) | .99 | 38 (5.4) | 21 (6.5) | .61 |
| Previous stroke | 32 (8.1) | 23 (10.3) | .44 | 38 (5.4) | 19 (5.9) | .90 |
| PAD | 14 (3.6) | 10 (4.5) | .72 | 13 (1.9) | 7 (2.2) | .94 |
| AF | 15 (3.8) | 9 (4.0) | .89 | 17 (2.4) | 14 (4.3) | .15 |
| LVEF, mean (SD), % | 56.4 (13.4) | 58.2 (11.4) | .10 | 59.5 (10.7) | 60.1 (10.7) | .44 |
| Treatment of diabetes | ||||||
| Lifestyle modification | 31 (7.9) | 13 (5.8) | .27 | NA | NA | NA |
| Oral hypoglycemic agent | 335 (85.0) | 187 (83.9) | NA | NA | ||
| Insulin | 28 (7.1) | 23 (10.3) | NA | NA | ||
| Laboratory data, mean (SD) | ||||||
| Fasting glucose, mg/dL | 151.1 (60.7) | 149.5 (59.2) | .76 | 114.4 (29.0) | 113.9 (29.6) | .83 |
| HbA1c, % | 7.2 (1.2) | 7.2 (1.3) | .99 | 5.8 (0.7) | 5.8 (0.5) | .64 |
| Creatinine, mg/dL | 1.3 (1.6) | 1.4 (1.8) | .49 | 1.1 (3.1) | 1.1 (1.7) | .92 |
| LDL cholesterol, mg/dL | 80.4 (33.3) | 83.7 (35.6) | .28 | 101.7 (41.2) | 96.9 (38.4) | .09 |
| Discharge medication | ||||||
| Aspirin | 384 (97.5) | 219 (98.2) | .75 | 685 (98.1) | 318 (98.1) | .99 |
| P2Y12 inhibitor | ||||||
| Any | 382 (97.0) | 220 (98.7) | .30 | 685 (98.1) | 316 (97.5) | .69 |
| Clopidogrel | 305 (79.8) | 177 (80.5) | 494 (72.1) | 240 (76.0) | ||
| Ticagrelor | 48 (12.6) | 22 (10.0) | 100 (14.6) | 39 (12.3) | ||
| Prasugrel | 29 (7.6) | 21 (9.5) | 91 (13.3) | 37 (11.7) | ||
| Oral anticoagulant | 18 (4.6) | 11 (4.9) | .99 | 28 (4.0) | 18 (5.6) | .34 |
| Statin | 364 (92.4) | 212 (95.1) | .26 | 677 (97.0) | 314 (96.9) | .95 |
| β-Blocker | 190 (48.2) | 105 (47.1) | .85 | 276 (39.5) | 139 (42.9) | .34 |
| ACE inhibitor or ARB | 241 (61.2) | 141 (63.2) | .67 | 381 (54.6) | 182 (56.2) | .68 |
Abbreviations: ACE, angiotensin-converting enzyme; AF, atrial fibrillation; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c, glycated hemoglobin A1c; IHD, ischemic heart disease; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not available; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; HbA1c to proportion of total hemoglobin, multiply by 0.01; and LDL cholesterol to millimoles per liter, multiply by 0.0259.
Among patients with diabetes, 394 were randomly assigned to undergo intravascular imaging–guided PCI and 223 were randomly assigned to undergo angiography-guided PCI. Among patients without diabetes, 698 were randomly assigned to undergo intravascular imaging–guided PCI and 324 were randomly assigned to undergo angiography-guided PCI. There were no significant differences in any baseline demographics, cardiovascular risk factors, initial presentation, laboratory findings, or medications at discharge between the 2 study arms regardless of diabetes status (Table 1).
Procedural Characteristics
eTable 2 in Supplement 1 presents baseline angiographic and procedural characteristics according to the presence of diabetes. Patients with diabetes were more likely to have in-stent restenosis lesions, multiple complex lesion profiles, and multivessel disease compared with patients without diabetes. For procedural characteristics, the mean (SD) number of stents was significantly greater (2.0 [1.1] vs 1.9 [0.9]; P = .01), the diameter of the stent was significantly smaller (3.1 [0.4] mm vs 3.2 [0.4] mm; P = .001), and the total length of the stent was significantly longer (58.3 [35.3] mm vs 53.8 [30.8] mm; P = .009) in patients with diabetes than those without diabetes. Patients with diabetes were more likely to need rotablation and less likely to achieve successful imaging-guided optimization than patients without diabetes (eFigure 2 in Supplement 1).
Baseline angiographic and procedural characteristics of the intravascular imaging guidance and angiography guidance according to diabetes are shown in Table 2. Regardless of the presence of diabetes, baseline complex lesion profiles were well balanced in the 2 study groups. Use of intravascular imaging vs angiography resulted in the more frequent use of adjunctive balloon dilation of the stent with noncompliant balloons in both patients with diabetes (290 [73.6%] vs 136 [61.0%]; P = .002) and patients without diabetes (512 [73.4%] vs 196 [60.5%]; P < .001). In addition, the mean (SD) procedural time was longer (patients with diabetes: 81.6 [43.4] minutes vs 66.7 [38.7] minutes; P < .001) and the mean (SD) volume of contrast was greater (patients with diabetes: 211.7 [116.0] mL vs 192.0 [108.4] mL; P = .04) in patients assigned to intravascular imaging–guided PCI compared with patients assigned to angiography-guided PCI (Table 2).
Table 2. Baseline Angiographic and Procedural Characteristics of Patients With Imaging-Guided or Angiography-Guided PCI by Diabetes Status.
| Characteristic | Patients with diabetes, No. (%) (n = 617) | Patients without diabetes, No. (%) (n = 1022) | ||||
|---|---|---|---|---|---|---|
| Imaging-guided PCI (n = 394) | Angiography-guided PCI (n = 223) | P value | Imaging-guided PCI (n = 698) | Angiography-guided PCI (n = 324) | P value | |
| Target lesion characteristics | ||||||
| Complex coronary lesions | ||||||
| True bifurcation (Medina 1,1,1/1,0,1/0,1,1) | 81 (20.6) | 45 (20.2) | .99 | 152 (21.8) | 81 (25.0) | .29 |
| Chronic total occlusion: ≥3 mo of occlusion | 77 (19.5) | 39 (17.5) | .60 | 143 (20.5) | 60 (18.5) | .52 |
| Unprotected left main CAD | 50 (12.7) | 19 (8.5) | .15 | 88 (12.6) | 35 (10.8) | .47 |
| Long coronary lesion: implanted stent length ≥38 mm | 228 (57.9) | 118 (52.9) | .27 | 389 (55.7) | 163 (50.3) | .12 |
| Multivessel PCI: ≥2 major coronary arteries treated | 151 (38.3) | 100 (44.8) | .13 | 258 (37.0) | 113 (34.9) | .57 |
| Multiple stents implanted: ≥3 stents per patient | 78 (19.8) | 49 (22.0) | .59 | 130 (18.6) | 48 (14.8) | .16 |
| In-stent restenosis lesion | 83 (21.1) | 40 (17.9) | .41 | 75 (10.7) | 38 (11.7) | .72 |
| Severely calcified lesion: encircling calcium in angiography | 66 (16.8) | 33 (14.8) | .60 | 91 (13.0) | 41 (12.7) | .94 |
| Ostial coronary lesion | 59 (15.0) | 32 (14.3) | .93 | 123 (17.6) | 37 (11.4) | .01 |
| No. of complex coronary lesions: ≥3 | 138 (35.0) | 76 (34.1) | .88 | 214 (30.7) | 77 (23.8) | .03 |
| Arteries with stenosis | ||||||
| 1-Vessel disease | 107 (27.2) | 64 (28.7) | .73 | 235 (33.7) | 120 (37.0) | .49 |
| 2-Vessel disease | 145 (36.8) | 75 (33.6) | 275 (39.4) | 126 (38.9) | ||
| 3-Vessel disease | 142 (36.0) | 84 (37.7) | 188 (26.9) | 78 (24.1) | ||
| Procedural characteristics | ||||||
| Total No. of target lesions treated, mean (SD) | 1.5 (0.7) | 1.6 (0.8) | .34 | 1.5 (0.7) | 1.4 (0.6) | .39 |
| Radial access | 271 (68.8) | 172 (77.1) | .03 | 556 (79.7) | 254 (78.4) | .70 |
| Intravascular imaging devices used | 390 (99.0) | 5 (2.2) | <.001 | 688 (98.6) | 8 (2.5) | <.001 |
| IVUS | 292 (74.9) [n = 390] | 5 (100) [n = 5] | 508 (73.8) | 8 (100) [n = 8] | ||
| OCT | 98 (25.1) [n = 390] | 0 [n = 5] | 180 (26.2) | 0 [n = 8] | ||
| Adjunctive noncompliant balloon used | 290 (73.6) | 136 (61.0) | .002 | 512 (73.4) | 196 (60.5) | <.001 |
| Rotablation used | 19 (4.8) | 9 (4.0) | .80 | 18 (2.6) | 7 (2.2) | .85 |
| Treatment devices used | ||||||
| DES | 380 (96.4) | 215 (96.4) | .99 | 684 (98.0) | 315 (97.2) | .58 |
| Drug-coated balloon angioplasty | 14 (3.6) | 8 (3.6) | 14 (2.0) | 9 (2.8) | ||
| Total No. of devices used per patient, mean (SD) | 2.0 (1.1) | 2.0 (1.1) | .57 | 1.9 (1.0) | 1.8 (0.9) | .07 |
| Dimensions of devices, mean (SD), mm | ||||||
| Mean diameter | 3.1 (0.4) | 3.1 (0.4) | .65 | 3.2 (0.4) | 3.1 (0.4) | <.001 |
| Total length | 57.6 (35.0) | 59.6 (35.7) | .50 | 55.0 (31.7) | 51.4 (28.4) | .07 |
| Volume of contrast media used, mean (SD), mL | 211.7 (116.0) | 192.0 (108.4) | .04 | 215.5 (119.9) | 194.9 (113.5) | .01 |
| Procedural time, mean (SD), min | 81.6 (43.4) | 66.7 (38.7) | <.001 | 79.5 (46.9) | 60.9 (35.2) | <.001 |
| Procedural success | 387 (98.2) | 220 (98.7) | .94 | 686 (98.3) | 320 (98.8) | .76 |
| Successful imaging-guided stent optimization | 145 (36.8) | NA | 351 (50.3) | NA | ||
Abbreviations: CAD, coronary artery disease; DES, drug-eluting stent; IVUS, intravascular ultrasonography; NA, not applicable; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
In quantitative coronary angiography data, pre-PCI reference mean (SD) diameter and post-PCI minimum lumen diameter were significantly smaller in patients with diabetes (0.4 [0.3] mm and 2.7 [0.5] mm) than patients without diabetes (0.5 [0.4] mm and 2.8 [0.5] mm) (eTable 3 in Supplement 1). No significant differences were observed in patients with diabetes regarding the pre-PCI and post-PCI quantitative coronary angiography data between the 2 study arms. However, in patients without diabetes, pre-PCI reference mean (SD) proximal diameter (3.3 [0.5] mm vs 3.2 [0.5] mm; P < .001) and post-PCI minimum lumen diameter (2.8 [0.5] mm vs 2.7 [0.5] mm; P = .001) were significantly larger in the imaging-guided PCI group than the angiography-guided PCI group.
End Points
Compared with patients without diabetes, those with diabetes had a significantly higher risk of TVF (12.7% vs 7.1%; HR, 1.86 [95% CI, 1.33-2.60]; P < .001) during a median (IQR) follow-up of 2.1 (1.4-3.0) years (eFigure 3 in Supplement 1). The incidence of TVF without procedure-related MI was significantly higher in patients with diabetes than patients without diabetes (HR, 2.37; 95% CI, 1.55-3.62; P < .001) (eFigure 3 in Supplement 1). These results were consistent even after conducting multivariable analyses, including covariables of age, sex, hypertension, dyslipidemia, history of PCI, chronic kidney insufficiency, acute coronary syndrome, left ventricular ejection fraction less than 50%, angiographic vessel disease, 3 or more complex coronary lesions, and study group (eTable 4 in Supplement 1).
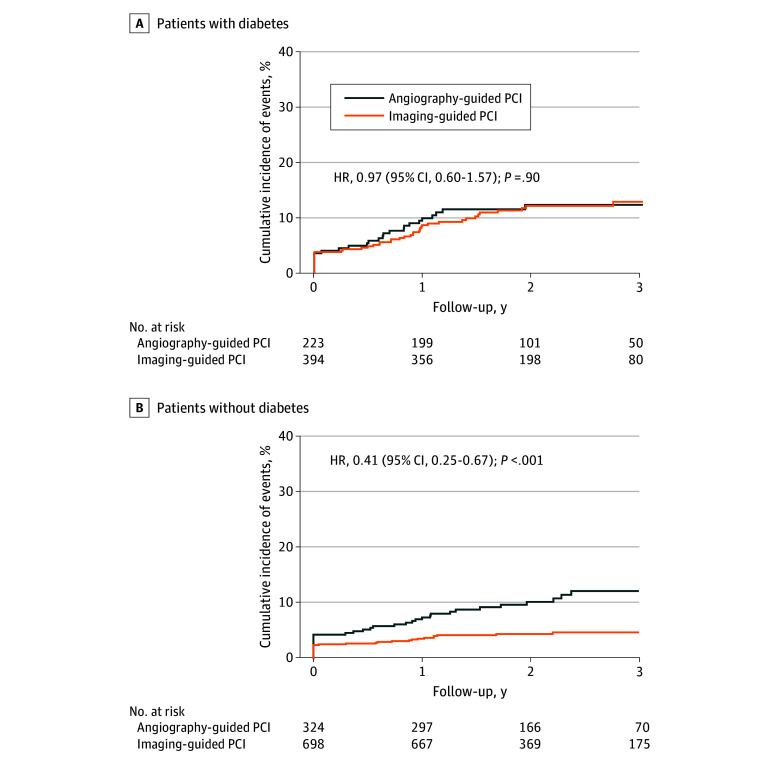
Among the population with diabetes, TVF occurred in 45 of 394 patients (12.9%) in the intravascular imaging–guided PCI group and 26 of 223 patients (12.3%) in the angiography-guided PCI group (HR, 0.97; 95% CI, 0.60-1.57; P = .90) (Table 3; Figure 1A). Among the population without diabetes, TVF occurred in 31 of 698 patients (4.7%) in the intravascular imaging–guided PCI group and 34 of 324 patients (12.2%) in the angiography-guided PCI group (HR, 0.41; 95% CI, 0.25-0.67; P < .001) (Table 3; Figure 1B). There was a significant interaction between presence of diabetes and use of intravascular imaging for the risk of TVF (P for interaction = .02).
Table 3. Primary and Secondary End Points in Patients With or Without Diabetesa .
| Patients with diabetes, No. (%) (n = 617) | Patients without diabetes, No. (%) (n = 1022) | P value for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Imaging-guided PCI (n = 394) | Angiography-guided PCI (n = 223) | HR (95% CI) | P value | Imaging-guided PCI (n = 698) | Angiography-guided PCI (n = 324) | HR (95% CI) | P value | ||
| Primary end point | |||||||||
| TVFb | 45 (12.9) | 26 (12.3) | 0.97 (0.60-1.57) | .90 | 31 (4.7) | 34 (12.2) | 0.41 (0.25-0.67) | <.001 | .02 |
| Secondary end point | |||||||||
| TVF without procedure-related MI | 32 (9.7) | 19 (9.2) | 0.94 (0.53-1.65) | .82 | 16 (2.6) | 21 (8.3) | 0.35 (0.18-0.67) | .001 | .02 |
| Cardiac death or target vessel–related MI | 31 (8.7) | 21 (9.9) | 0.83 (0.47-1.44) | .50 | 22 (3.4) | 22 (7.6) | 0.46 (0.25-0.83) | .01 | .15 |
| All-cause death | 25 (8.0) | 15 (7.4) | 0.93 (0.49-1.76) | .83 | 17 (3.7) | 13 (5.8) | 0.60 (0.29-1.23) | .16 | .37 |
| Cardiac death | 13 (4.1) | 9 (4.2) | 0.81 (0.35-1.90) | .63 | 3 (0.4) | 8 (3.4) | 0.17 (0.05-0.65) | .01 | .05 |
| MI | 24 (7.1) | 15 (7.2) | 0.90 (0.47-1.72) | .76 | 19 (3.0) | 17 (5.6) | 0.51 (0.27-0.99) | .04 | .23 |
| Target vessel–related MI | 19 (5.0) | 15 (7.2) | 0.71 (0.36-1.40) | .32 | 19 (3.0) | 15 (4.6) | 0.58 (0.30-1.15) | .12 | .68 |
| Spontaneous MI | 5 (1.4) | 8 (4.0) | 0.35 (0.11-1.06) | .06 | 3 (0.7) | 1 (0.3) | 1.38 (0.14-13.26) | .78 | .28 |
| Procedure-related MI | 14 (3.6) | 8 (3.6) | 0.99 (0.42-2.36) | .98 | 16 (2.3) | 14 (4.3) | 0.53 (0.26-1.08) | .08 | .27 |
| Repeat revascularization | 32 (10.4) | 14 (7.4) | 1.29 (0.69-2.41) | .43 | 23 (4.2) | 18 (6.9) | 0.58 (0.31-1.08) | .09 | .08 |
| Target vessel revascularization | 19 (5.7) | 12 (6.1) | 0.88 (0.43-1.81) | .72 | 13 (2.2) | 13 (5.1) | 0.46 (0.21-0.98) | .04 | .23 |
| Target lesion revascularization | 15 (4.6) | 11 (5.2) | 0.75 (0.34-1.63) | .47 | 9 (1.5) | 9 (3.7) | 0.46 (0.18-1.15) | .10 | .42 |
| Definite stent thrombosis | 1 (0.3) | 4 (1.8) | 0.14 (0.02-1.26) | .08 | 0 | 0 | NA | NA | NA |
| Contrast-induced nephropathy | 14 (3.6) | 10 (4.5) | 0.77 (0.34-1.73) | .53 | 12 (1.7) | 4 (1.2) | 1.52 (0.49-4.72) | .47 | .35 |
Abbreviations: HR, hazard ratio; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; TVF, target vessel failure.
Cumulative incidence of events presented as Kaplan-Meier estimates.
Primary end point was TVF, defined as a composite of cardiac death, target vessel–related MI, and target vessel revascularization.
Figure 1. Cumulative Incidences of Target Vessel Failure (TVF) Between Angiography-Guided vs Imaging-Guided Percutaneous Coronary Intervention (PCI) by Diabetes Status.
There was a significant interaction between presence of diabetes and use of intravascular imaging for the risk of TVF (P for interaction = .02). HR indicates hazard ratio.
Outcomes According to Imaging-Guided Stent Optimization and Glycemic Control in Patients With Diabetes
The mean (SD) HbA1c level in patients with diabetes was 7.2% (1.2%) (eFigure 4 in Supplement 1). There was an association between baseline HbA1c level and risk of TVF (HR [per 1% increase], 1.16; 95% CI, 1.01-1.32; P = .03) (eFigure 4 in Supplement 1). Among the population with diabetes, poor glycemic control (HbA1c level >7.5%) was associated with significantly higher risk of TVF (17.9% vs 10.3%; HR, 1.69 [95% CI, 1.06-2.71]; P = .03) and TVF without procedure-related MI (14.4% vs 7.3%; HR, 1.91 [95% CI, 1.10-3.32]; P = .02) compared with patients with good glycemic control (HbA1c level ≤7.5%) (eFigure 5 in Supplement 1). Patients with poor glycemic control at baseline consistently showed higher follow-up HbA1c levels at 6 and 12 months than patients with good glycemic control (eFigure 6 in Supplement 1). Detailed intravascular imaging parameters between optimized and unoptimized lesions in patients with diabetes are shown in eTable 5 in Supplement 1. Compared with unoptimized lesions, optimized lesions showed a significantly larger mean (SD) MSA after PCI (6.2 [1.9] vs 4.5 [1.3]; P < .001). When stratifying patients with diabetes into intravascular imaging–guided stent optimization and glycemic control status, there was a marginal interaction between glycemic control and intravascular imaging–guided stent optimization for the primary end point (P for interaction = .05). Among these patients, only patients with good glycemic control and stent optimization confirmed by intravascular imaging showed a significantly lower risk of TVF compared with patients with poor glycemic control and those who underwent angiography-guided PCI only or did not achieve stent optimization by intravascular imaging (HR, 0.31; 95% CI, 0.12-0.82; P = .02) (Figure 2).
Figure 2. Comparative Hazard Ratios (HRs) of Target Vessel Failure by Glycemic Control Status and Imaging-Guided Stent Optimization for Patients With Diabetes Who Underwent Complex Percutaneous Coronary Intervention .
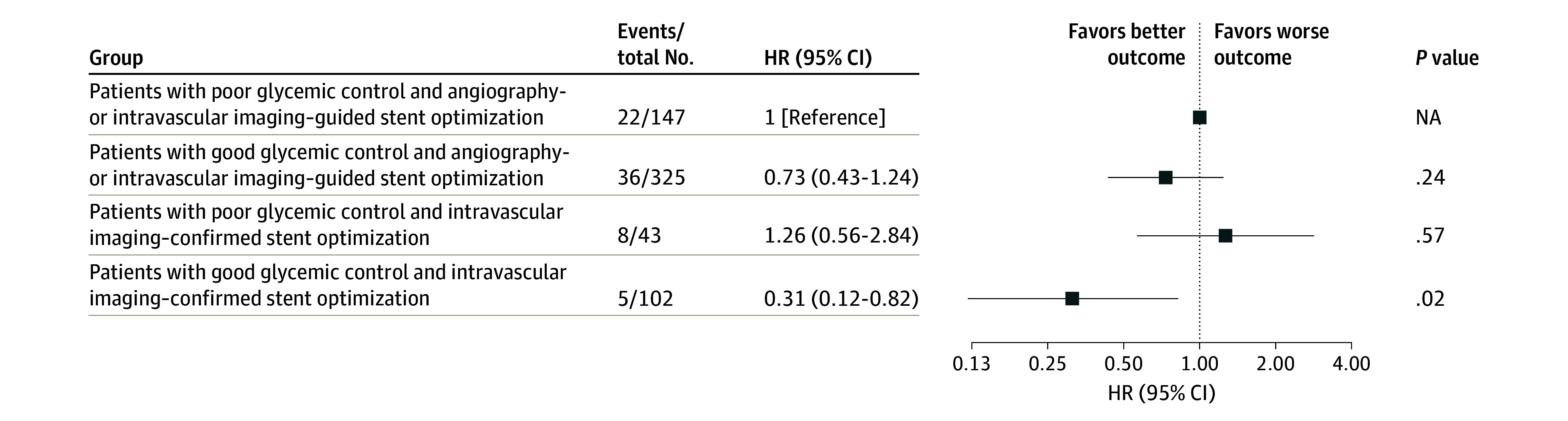
Error bars represent 95% CIs. Squares represent HRs. NA indicates not available.
Discussion
This subgroup analysis evaluated differential effects of intravascular imaging guidance on patients with complex coronary artery lesions according to presence of diabetes. There were several key findings of this study. First, patients with diabetes had a higher risk of TVF than patients without diabetes after complex PCI. Second, intravascular imaging–guided PCI for complex coronary artery lesions was associated with a lower incidence of TVF compared with angiography-guided PCI in patients without diabetes but not in patients with diabetes. There was a significant interaction between diabetes and intravascular imaging guidance for the risk of TVF. Third, among patients with diabetes, improved clinical outcomes were observed only in patients who achieved both good glycemic control and intravascular imaging–guided stent optimization.
Ischemic Risk After Complex PCI in Patients With Diabetes
The role of diabetes in the pathophysiological progression of coronary atherosclerosis is well characterized.21 A 3-dimensional IVUS follow-up study showed plaque phenotype with a higher risk of future cardiac events and further progression of atherosclerosis was observed in patients with diabetes than in patients without diabetes despite reaching a similar level of low-density lipoprotein cholesterol.22 Although numerous previous studies showed that patients with diabetes had higher numbers of ischemic adverse events after PCI than patients without diabetes even in the contemporary DES era,1,2,3 data focusing on patients who underwent complex PCI are scarce. In a previous pooled analysis of 18 randomized clinical trials, outcomes were comparable between patients with and without diabetes after PCI for simple lesions, whereas patients with diabetes with complex lesions had significantly higher rates of ischemic events after DES implantation than patients without diabetes with complex lesions.23 Considering the previous results, the effects of diabetes are likely to be maximized in patients undergoing complex PCI. In line with this background, the present study found that diabetes was independently associated with higher risks of ischemic events, including individual hard end points (cardiac death, MI, and stent thrombosis) compared with no diabetes. Therefore, the results emphasize that patients with diabetes still experience a higher risk of ischemic events, particularly for complex PCI, when treated with current-generation DES, and efforts to improve the clinical outcomes in this high-risk subgroup are necessary.
Differential Effects of Intravascular Imaging Guidance During Complex PCI According to Diabetes Status
The subgroup analyses of the ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions) and IVUS-XPL (Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions) trials consistently showed that IVUS-guided PCI resulted in lower risk of ischemic events for patients with and without diabetes.15,16 Furthermore, recent large trials, ILUMIEN IV (Optical Coherence Tomography (OCT) Guided Coronary Stent Implantation Compared to Angiography: A Multicenter Randomized Trial in PCI) and OCTOBER (European Trial on Optical Coherence Tomography Optimized Bifurcation Event Reduction), also showed that the effects of OCT-guided PCI were not different according to the presence of diabetes.24,25 However, the current study found that the benefits of intravascular imaging–guided complex PCI were observed in patients without diabetes but not in patients with diabetes, with significant interactions between diabetes and intravascular imaging guidance. This discrepancy might be due to the unique feature of the RENOVATE-COMPLEX-PCI trial. In contrast to the previous trials focusing on the effects of intravascular imaging, the RENOVATE-COMPLEX-PCI trial exclusively enrolled patients with various types of complex coronary artery lesions. Furthermore, the most contemporary validated criteria for stent optimization using IVUS or OCT guidance were adopted in the current study.14 In high-risk patients with diabetes, increased coronary lesion complexity may lead to a higher occurrence of ischemic events after PCI, and complex anatomical profiles might not be overcome despite the use of intravascular imaging during PCI. The current study revealed that patients with diabetes who were assigned to the intravascular imaging–guided PCI group were less likely to achieve stent optimization confirmed by contemporary criteria for intravascular imaging compared with patients without diabetes who were assigned to the intravascular imaging–guided PCI group. These results support the importance of standardized stent optimization criteria for intravascular imaging, especially in patients with diabetes and complex coronary artery lesions undergoing PCI. Furthermore, these results are consistent with findings from several randomized clinical trials and meta-analyses that CABG was superior to PCI for minimizing the risk of ischemic events in patients with diabetes and complex CAD.8,9,10,11 Therefore, it is necessary to carefully decide the revascularization method when encountering complex coronary artery lesions with diabetes, and CABG might be a good option for this subgroup.
Effect of Glycemic Control and Imaging-Guided Stent Optimization on Clinical Outcomes of Patients With Diabetes
HbA1c level served as an indicator of glycemic control status over the past 8 to 12 weeks; elevated HbA1c level was associated with increased risk of cardiovascular diseases in patients with diabetes.26 In particular, in the case of diabetes with proven CAD after PCI, several studies consistently showed that intensive glycemic status control might be associated with reduced risk of ischemic events.27,28,29 In line with the previous findings, we found that patients with diabetes with poor glycemic control had worse outcomes than patients with good glycemic control. To identify high-risk patients with diabetes who are more likely to experience good outcomes after complex PCI, we further stratified these patients based on glycemic control status and achievement of intravascular imaging–guided stent optimization. Only patients with diabetes with good glycemic control and who met intravascular imaging–guided stent optimization criteria had a reduced risk of future ischemic events.
These findings suggest that strict secondary prevention, including glycemic control and meticulous procedural optimization using intravascular imaging guidance, should be pursued to improve clinical outcomes when performing PCI for complex coronary lesions in high-risk patients with diabetes. However, the results of the present study should be interpreted primarily as hypothesis generating because of the exploratory nature of the analysis. Therefore, a well-designed larger study focusing on patients with diabetes with complex CAD is warranted to confirm these findings.
Limitations
The limitations of this study should be considered. First, although prespecified, this subgroup analysis was conducted within a randomized clinical trial. Consequently, the analysis may lack sufficient statistical power to detect differences in the primary end points between study groups for patients with and without diabetes. Second, due to the open-label design of the RENOVATE-COMPLEX-PCI trial, there remains a possibility of bias in the identification of events. However, we implemented measures to minimize this risk, such as using predefined criteria for end point analysis, conducting angiographic and imaging assessments at core laboratories, and having a blinded committee adjudicate clinical events. Third, the assessment of stent optimization in the angiography-only group relied solely on quantitative coronary angiography since the patients in this group did not undergo intravascular imaging.
Fourth, results from multiple strata of nonrandomized subgroups were only hypothesis generating coupled with low statistical power. Fifth, detailed information about oral hypoglycemic agents used in patients with diabetes and duration of diabetes information were not available in the current study. Considering the benefits of sodium glucose cotransporter-2 inhibitors in patients with heart failure, the lack of information about oral hypoglycemic agents might be a major missing point in interpreting results for patients with diabetes. Furthermore, the control of other risk factors, particularly hypertension, may have played an important role in disease progression and occurrence of clinical events, but these data were also not available. Sixth, although the current study applied the most contemporary criteria for stent optimization using IVUS or OCT, cutoff values of MSA were not validated in patients with diabetes. Seventh, the RENOVATE-COMPLEX-PCI trial exclusively enrolled patients in Korea ; therefore, the current findings might not be extrapolated to other populations.
Conclusions
In this secondary analysis of the RENOVATE-COMPLEX-PCI randomized clinical trial, intravascular imaging guidance was associated with a decreased risk of TVF compared with angiography guidance in patients without diabetes who underwent PCI for complex coronary lesions. However, there was no significant difference in the risk of TVF between intravascular imaging–guided and angiography-guided complex PCI in patients with diabetes. For patients with diabetes undergoing complex PCI, it is crucial to focus on both stent optimization through intravascular imaging and glycemic control to improve outcomes. However, these results must be interpreted cautiously because of the exploratory nature and small sample size of the analysis.
eAppendix
eMethods.
eTable 1. Comparison of Baseline Characteristics According to the Presence of Diabetes Mellitus
eTable 2. Baseline Angiographic and Procedural Characteristics According to the Presence of Diabetes Mellitus
eTable 3. Lesion-Level Analysis of Quantitative Coronary Angiography According to Diabetes Mellitus and Allocation Group
eTable 4. Primary and Secondary Endpoints According to the Presence of Diabetes Mellitus
eTable 5. Lesion-Level Analysis of Intravascular Imaging Guided Optimization in Patients With Diabetes Mellitus
eFigure 1. Study Flowchart
eFigure 2. Proportion of Achievement for Pre-Specified Imaging Optimization Criteria in Imaging-Guided PCI Arm According to Diabetes Mellitus
eFigure 3. Cumulative Incidences of Endpoints According to Presence of Diabetes Mellitus
eFigure 4. Distribution of Hemoglobin A1c and Its Association With Primary Endpoint in Diabetic Patients
eFigure 5. Cumulative Incidences of Study Outcomes According to Glycemic Control in Diabetic Patients
eFigure 6. Serial Changes in HbA1c According to Baseline Glycemic Control Status
eReferences
Trial Protocol
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Ploumen EH, Pinxterhuis TH, Zocca P, et al. Impact of prediabetes and diabetes on 3-year outcome of patients treated with new-generation drug-eluting stents in two large-scale randomized clinical trials. Cardiovasc Diabetol. 2021;20(1):217. doi: 10.1186/s12933-021-01405-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chichareon P, Modolo R, Kogame N, et al. Association of diabetes with outcomes in patients undergoing contemporary percutaneous coronary intervention: pre-specified subgroup analysis from the randomized GLOBAL LEADERS study. Atherosclerosis. 2020;295:45-53. doi: 10.1016/j.atherosclerosis.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Park GM, Han S, et al. Impact of diabetes mellitus in patients undergoing contemporary percutaneous coronary intervention: results from a Korean nationwide study. PLoS One. 2018;13(12):e0208746. doi: 10.1371/journal.pone.0208746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi: 10.1186/s12933-018-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara T, Sotomi Y, Tsujimura T, et al. Impact of diabetes mellitus on the early-phase arterial healing after drug-eluting stent implantation. Cardiovasc Diabetol. 2020;19(1):203. doi: 10.1186/s12933-020-01173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki M, Otake H, Shinke T, et al. Vascular responses in patients with and without diabetes mellitus after everolimus-eluting stent implantation. Circ J. 2014;78(9):2188-2196. doi: 10.1253/circj.CJ-13-1540 [DOI] [PubMed] [Google Scholar]
- 7.Sakata K, Waseda K, Kume T, et al. Impact of diabetes mellitus on vessel response in the drug-eluting stent era: pooled volumetric intravascular ultrasound analyses. Circ Cardiovasc Interv. 2012;5(6):763-771. doi: 10.1161/CIRCINTERVENTIONS.111.962878 [DOI] [PubMed] [Google Scholar]
- 8.Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55(5):432-440. doi: 10.1016/j.jacc.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 9.Farkouh ME, Domanski M, Sleeper LA, et al. ; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 10.Kappetein AP, Head SJ, Morice MC, et al. ; SYNTAX Investigators . Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013;43(5):1006-1013. doi: 10.1093/ejcts/ezt017 [DOI] [PubMed] [Google Scholar]
- 11.Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391(10124):939-948. doi: 10.1016/S0140-6736(18)30423-9 [DOI] [PubMed] [Google Scholar]
- 12.Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18-e114. doi: 10.1161/CIR.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 13.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 14.Räber L, Mintz GS, Koskinas KC, et al. ; ESC Scientific Document Group . Clinical use of intracoronary imaging, part 1: guidance and optimization of coronary interventions: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39(35):3281-3300. doi: 10.1093/eurheartj/ehy285 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE Trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi: 10.1016/j.jacc.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 16.Hong SJ, Kim BK, Shin DH, et al. ; IVUS-XPL Investigators . Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314(20):2155-2163. doi: 10.1001/jama.2015.15454 [DOI] [PubMed] [Google Scholar]
- 17.Choi KH, Song YB, Lee JM, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12(7):607-620. doi: 10.1016/j.jcin.2019.01.227 [DOI] [PubMed] [Google Scholar]
- 18.Lee JM, Choi KH, Song YB, et al. ; RENOVATE-COMPLEX-PCI Investigators . Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med. 2023;388(18):1668-1679. doi: 10.1056/NEJMoa2216607 [DOI] [PubMed] [Google Scholar]
- 19.Yamawaki M, Terashita D, Takahashi H, et al. ; J-REVERSE Investigators . Impact of diabetes mellitus on intravascular ultrasound-guided provisional stenting in coronary bifurcation lesions J-REVERSE sub-study. J Interv Cardiol. 2016;29(6):576-587. doi: 10.1111/joic.12353 [DOI] [PubMed] [Google Scholar]
- 20.Al-Badri A, Hashmath Z, Oldland GH, et al. Poor glycemic control is associated with increased extracellular volume fraction in diabetes. Diabetes Care. 2018;41(9):2019-2025. doi: 10.2337/dc18-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittl JA. Percutaneous coronary interventions in the diabetic patient: where do we stand? Circ Cardiovasc Interv. 2015;8(4):e001944. doi: 10.1161/CIRCINTERVENTIONS.114.001944 [DOI] [PubMed] [Google Scholar]
- 22.Kovarnik T, Chen Z, Mintz GS, et al. Plaque volume and plaque risk profile in diabetic vs. non-diabetic patients undergoing lipid-lowering therapy: a study based on 3D intravascular ultrasound and virtual histology. Cardiovasc Diabetol. 2017;16(1):156. doi: 10.1186/s12933-017-0637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kedhi E, Généreux P, Palmerini T, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus: analysis from 18 pooled randomized trials. J Am Coll Cardiol. 2014;63(20):2111-2118. doi: 10.1016/j.jacc.2014.01.064 [DOI] [PubMed] [Google Scholar]
- 24.Ali ZA, Landmesser U, Maehara A, et al. ; ILUMIEN IV Investigators . Optical coherence tomography-guided versus angiography-guided PCI. N Engl J Med. 2023;389(16):1466-1476. doi: 10.1056/NEJMoa2305861 [DOI] [PubMed] [Google Scholar]
- 25.Holm NR, Andreasen LN, Neghabat O, et al. ; OCTOBER Trial Group . OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med. 2023;389(16):1477-1487. doi: 10.1056/NEJMoa2307770 [DOI] [PubMed] [Google Scholar]
- 26.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412. doi: 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang JK, Lee SH, Song YB, et al. Glycemic control status after percutaneous coronary intervention and long-term clinical outcomes in patients with type 2 diabetes mellitus. Circ Cardiovasc Interv. 2017;10(4):e004157. doi: 10.1161/CIRCINTERVENTIONS.116.004157 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Li X, Zhang Y, et al. Impact of glycemic control status on patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2020;20(1):36. doi: 10.1186/s12872-020-01339-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma PK, Agarwal S, Ellis SG, et al. Association of glycemic control with mortality in patients with diabetes mellitus undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(4):503-509. doi: 10.1161/CIRCINTERVENTIONS.113.001107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix
eMethods.
eTable 1. Comparison of Baseline Characteristics According to the Presence of Diabetes Mellitus
eTable 2. Baseline Angiographic and Procedural Characteristics According to the Presence of Diabetes Mellitus
eTable 3. Lesion-Level Analysis of Quantitative Coronary Angiography According to Diabetes Mellitus and Allocation Group
eTable 4. Primary and Secondary Endpoints According to the Presence of Diabetes Mellitus
eTable 5. Lesion-Level Analysis of Intravascular Imaging Guided Optimization in Patients With Diabetes Mellitus
eFigure 1. Study Flowchart
eFigure 2. Proportion of Achievement for Pre-Specified Imaging Optimization Criteria in Imaging-Guided PCI Arm According to Diabetes Mellitus
eFigure 3. Cumulative Incidences of Endpoints According to Presence of Diabetes Mellitus
eFigure 4. Distribution of Hemoglobin A1c and Its Association With Primary Endpoint in Diabetic Patients
eFigure 5. Cumulative Incidences of Study Outcomes According to Glycemic Control in Diabetic Patients
eFigure 6. Serial Changes in HbA1c According to Baseline Glycemic Control Status
eReferences
Trial Protocol
Nonauthor Collaborators
Data Sharing Statement