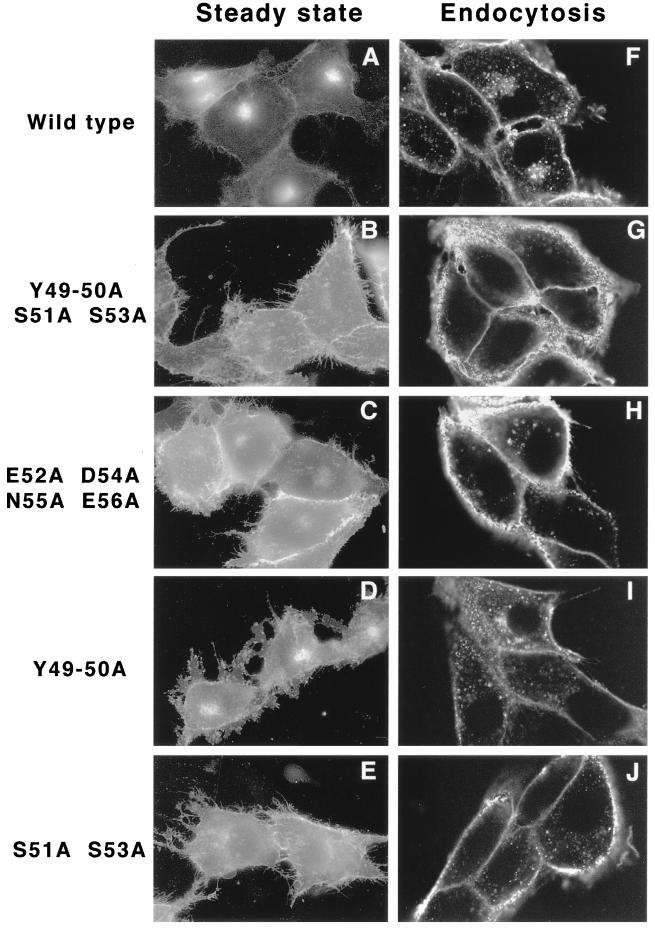
FIG. 6.
(A through E) Transient transfection of Us9 alanine scanning mutant constructs. PK15 cells grown on glass coverslips were transfected by the calcium phosphate method with plasmids encoding Us9–EGFP (A), Us9(Y49–50A S51A S53A)–EGFP (B), Us9(E52A D54A N55A E56A)–EGFP (C), Us9(Y49–50A)–EGFP (D), and Us9(S51A S53A)–EGFP (E). At 48 h posttransfection, the localization of the various Us9–EGFP fusion proteins was detected by fluorescence microscopy. (F through J) Internalization of alanine scanning Us9 mutant proteins from the cell surface. PK15 cells stably expressing wild-type Us9–EGFP (F), Us9(Y49–50A S51A S53A)–EGFP (G), Us9(E52A D54A N55A E56A)–EGFP (H), Us9(Y49–50A)–EGFP (I), and Us9(S51A S53A)–EGFP (J) fusion proteins were plated on glass coverslips. The coverslips were incubated at 37°C in medium containing a 1:75 dilution of polyclonal GFP-specific antiserum. After 1 h, the cells were fixed and permeabilized, and the localization of the GFP-specific antibodies was detected by indirect immunofluorescence followed by confocal microscopy. Only the red fluorescence of the Alexa 568-conjugated goat anti-rabbit IgG secondary antibody is shown.