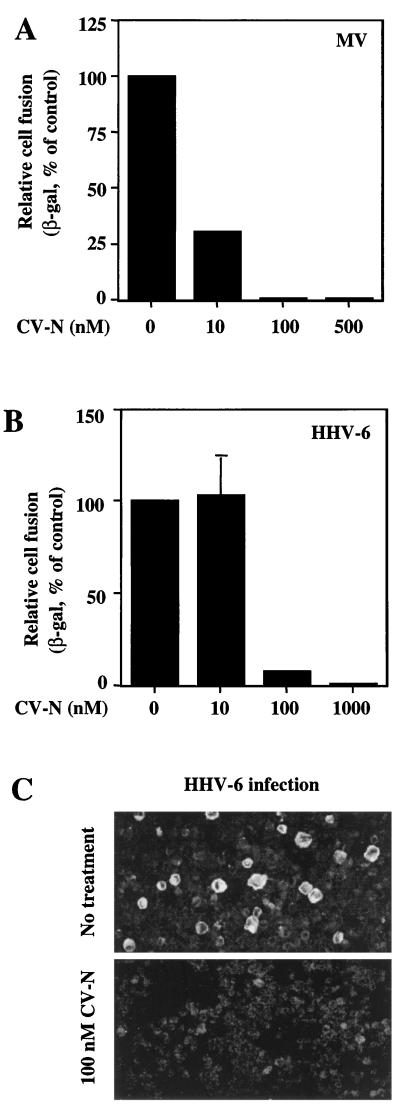
FIG. 7.
Inhibitory effects of CV-N on MV and HHV-6. For the experiments shown in panels A and B, the vaccinia virus-based reporter gene cell fusion assays were used. The indicated CV-N concentrations represent those in the final fusion mixtures. (A) Effect of CV-N on MV envelope glycoprotein-mediated fusion. The background β-Gal activity value (0.6), obtained with the effector cells expressing the hemagglutinin protein alone, was subtracted from each value obtained with effector cells expressing both hemagglutinin and fusion glycoproteins. The β-Gal activity (72.4) obtained from MV envelope glycoproteins-mediated fusion in absence of CV-N was defined as 100%. (B) Effect of CV-N on HHV-6-mediated cell fusion. As a background control, the β-Gal activities from effector cells (PBMCs) and target cells (HeLa) incubated alone were combined, and this value (2.48) was subtracted from each point to give data shown. The β-Gal activity (6.24) obtained from fusion in absence of CV-N was defined as 100%. In panels A and B, β-Gal values represent the means of duplicate samples; error bars indicate standard deviation of the mean values obtained from duplicate samples. (C) Effect of CV-N on HHV-6 infection. Infection of PBMCs was performed in the absence (top) or continuous presence (bottom) of 100 nM CV-N. After 3 days, the cells were assayed by immunofluorescence microscopy for the presence of the HHV-6 glycoproteins gp102 and gp116. The large, brightly stained cells in the top panel are typical of HHV-6 infection.