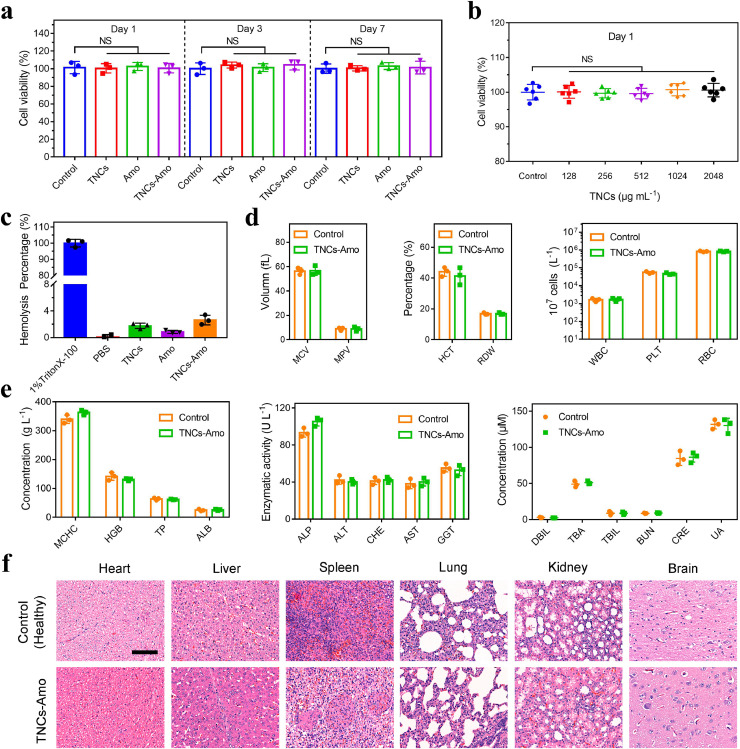
Fig. 4.
Biosafety assay. (a) The cell viability of A549 cells with TNCs, Amo, and TNCs–Amo was tested by LDH after 1, 3, and 7 days. (b) Cell viability of A549 cells treated with TNCs at various concentrations after co-culturing for 1 day (n = 5 independent experiments). (c) Hemolysis test of samples. (d, e) Biosafety study of TNCs–Amo in piglets. Hematology (mean corpuscular volume [MCV], mean platelet volume [MPV], hematocrit [HCT], red cell distribution width [RDW], white blood cell [WBC], platelets [PLT], red blood cell [RBC], mean corpuscular hemoglobin concentration [MCHC], and hemoglobin [HGB]), liver function (total protein [TP], albumin [ALB], alkaline phosphatase [ALP], alanine aminotransferase [ALT], cholinesterase [CHE], aspartic transaminase [AST], γ-glutamyl transpeptidase [GGT], and direct bilirubin [DBIL]), and kidney function (urea nitrogen [BUN], creatinine [CRE], and uric acid [UA]) were tested in piglets after nebulization with TNCs–Amo. (f) Hematoxylin and eosin (H&E)-stained images of tissue sections from piglets. Scale bar, 100 μm. In (a), (c), (d) and (e), the experiments were repeated three times independently with similar results.