Table 2.
Substrate scope for synthesizing aryl-pyrroloindoles 7 bearing axial and central chiralitya.
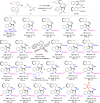 |
a Reaction conditions: 1 (0.1 mmol), 6 (0.12 mmol), (R)-4a (15 mol%), DCM/EtOAc (4:1 v/v, 5 mL), 20 °C for 10–90 h. Isolated yields were provided and the dr values were determined by 1H NMR. The ee values referred to those of the major diastereomers and were determined by HPLC analysis on a chiral stationary phase.
