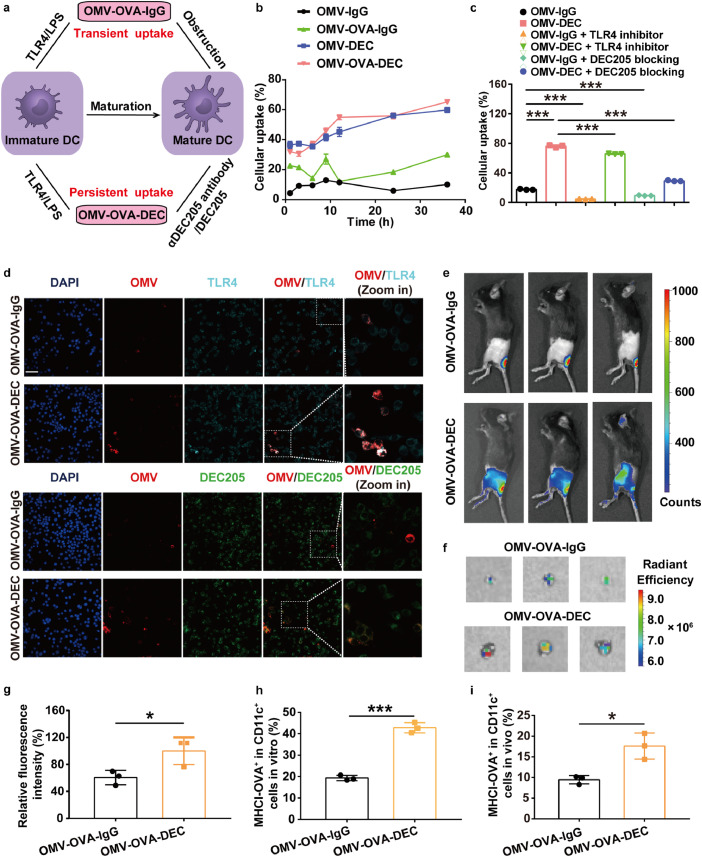
Fig. 2.
Improved antigen delivery of OMV-DEC-based nanocarrier mediated by DEC205/αDEC205 antibody interaction. (a) Schematic illustration depicting the uptake enhancement mediated by αDEC205 antibody modification, due to the transition from transient uptake of OMV-OVA-IgG to persistent uptake of OMV-OVA-DEC by DCs. (b) BMDC uptake of the indicated OMV-based nanovaccines constructed by PE-labeled αDEC205 antibody and IgG isotype, as measured by flow cytometry (n = 3). (c) BMDC uptake of PE-labeled OMV-IgG or OMV-DEC at 12 h with or without pre-blocking with TLR4 inhibitor (1 µM TAK-242) or DEC205 blocking antibody (5 µg/mL αDEC205 antibody) for 1 h (n = 3). (d) Laser scanning confocal microscopy images of DC2.4 cells after 1 h incubation with the indicated OMV-based nanovaccines. OMV-based nanovaccines were labeled with Dil (red). DEC205 was detected with a PE-labeled antibody (green), and TLR4 was detected with a FITC-labeled antibody (cyan). Scale bar, 50 µm. (e)–(g) Lymph node drainage of OMV-OVA-IgG and OMV-OVA-DEC labeled with Cy5.5-labeled OVA at 6 h after subcutaneous administration at the base of tail. Lymph node accumulation was evaluated visually by examining the Cy5.5 fluorescence in vivo (e) and ex vivo (f), and quantitatively analyzed (g). The fluorescence intensity in the OMV-OVA-DEC group was set to 100% (n = 3). (h) and (i) Antigen presentation efficiency in DCs as examined by flow cytometry using an OVA257-264-specific MHCI complex (MHCI-OVA) antibody at 12 h after incubation in BMDCs in vitro (h) or 6 h after subcutaneous vaccination in vivo (i) (n = 3). One-way ANOVA with a Tukey post-hoc test was used for statistical analysis. Data are shown as mean ± SD.*, P < 0.05; ⁎⁎⁎, P < 0.001.