TABLE 2.
Prediction of BBB permeability of statins and their involvements in brain tumor clinical trials
| Drug name | SMILES | 2D depiction | LogBB value[15] | Clinical trials | Tumor type | Interventions |
|---|---|---|---|---|---|---|
| Simvastatin (Zocor®) | CCC(C)(C)C(=O)O[C@H]1C [C@@H](C)C=C2C=C[C@H] (C)[C@H](CC[C@@H]3C [C@@H](O)CC(=O)O3)[C @H]21 |

|
−0.20879 | NCT01764451 | Cerebral Cavernous Hemangioma | Simvastatin |
| NCT02104193 | Brain Metastases | Simvastatin + Radiation therapy | ||||
| Atorvastatin (Lipitor) | CC(C)c1c(C(=O)Nc2ccccc2) c(-c2ccccc2)c(-c2ccc(F) cc2)n1CC[C@H](O)C [C@H](O)CC(=O)O |

|
−1.34826 | NCT02603328 | Cerebral Cavernous Hemangioma | Atorvastatin |
| NCT02029573 | Glioblastoma Multiforme | Atorvastatin + Temozolomide + Radiotherapy | ||||
| Rosuvastatin (Crestor) | CC(C)c1nc(N(C)S(C)(=O)=O) nc(-c2ccc(F)cc2)c1C=CC(O) C[C@@H](O)CC(=O)O |
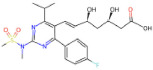
|
−1.14247 | N | ||
| Fluvastatin (Lescol) | CC(C)n1c(C=CC(O)C[C@H](O) CC(=O)O)c(-c2ccc(F) cc2)c2ccccc21 |

|
−1.04942 | N | ||
| Lovastatin (Mevacor) | CC[C@H](C)C(=O)O[C@H]1C [C@@H](C)C=C2C=C[C@H] (C)[C@H](CO[C@@H]3C[C@ @H](O)CC(=O)O3)[C@H]21 |
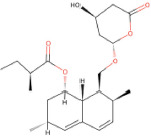
|
−0.30716 | NCT04297033 | Cerebral Arteriovenous Malformation | Lovastatin |
| Pitavastatin (Livalo) | O=C(O)C[C@H](O)CC(O)C=C c1c(C2CC2)nc2ccccc2c1-c1ccc(F)cc1 |
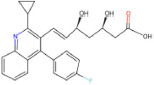
|
−1.12192 | NCT05977738 | Glioblastoma Multiforme; Recurrent Glioblastoma | Pitavastatin calcium |
| Pravastatin (Pravachol) | CC[C@H](C)C(=O)O[C@H]1C [C@H](O)C=C2C=C[C@H] (C)[C@H](CC[C@@H](O)C [C@@H](O)CC(=O)O)[C@H]21 |
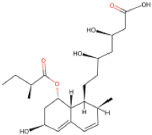
|
−0.91495 | N |
Abbreviations: BBB, blood-brain barrier; SMILES, simplified molecular-input line-entry system; LogBB value, a logarithmic ratio of the concentration of a drug in the brain to its concentration in the blood.
Clinical trial data were assessed through clinicaltrials.gov at March 21, 2024.
