Abstract
Food-triggered anaphylaxis can encompass a variety of systemic and intestinal symptoms. Murine-based and clinical studies have revealed a role for histamine and H1R and H2R-pathway in the systemic response, however, the molecular processes that regulate the gastrointestinal (GI) response are not as well defined. In the present study, by utilizing an IgE-mast cell (MC)-dependent experimental model of oral antigen-induced anaphylaxis, we define the intestinal epithelial response during a food-induced anaphylactic reaction. We show that oral allergen-challenge stimulates a rapid dysregulation of intestinal epithelial transcellular and paracellular transport that was associated with the development of secretory diarrhea. Allergen-challenge induced (i) a rapid intestinal epithelial Cftr-dependent Cl− secretory response and (ii) paracellular macromolecular leak that was associated with modification in epithelial intercellular junction proteins claudin-1, 2, 3 and 5, E-cadherin and desmosomal cadherins. OVA-induced Cftr-dependent Cl− secretion and junctional protein degradation was rapid occurring and was sustained for 72 h following allergen-challenge. Blockade of both the proteolytic activity and Cl− secretory response was required to alleviate intestinal symptoms of food-induced anaphylaxis. Collectively, these data suggest that the GI symptom of food-induced anaphylactic reaction, secretory diarrhea, is a consequence of CFTR-dependent Cl− secretion and proteolytic activity.
Introduction
Severe food allergy-related reactions, termed food-triggered anaphylaxis, are serious life threatening reactions responsible for 30,000-120,000 emergency department visits, 2,000 - 3000 hospitalizations, and approximately 150 deaths per year in the United States1,2. The onset of symptoms are variable, occurring within seconds to a few hours following exposure to the casual dietary allergen, and often affects multiple organ systems including gastrointestinal (GI), cutaneous, respiratory and cardiovascular3. Cutaneous symptoms (urticaria and angioedema) are the most common, occurring in approximately 80% of cases and GI symptoms occur in as much as 40% cases, which include cramping, abdominal pain, nausea, emesis and diarrhea4. Recently, there has been emerging clinical data indicating a link between GI manifestations with the more severe anaphylactic phenotype including hypotension and hypoxia5–9. Clinical and experimental analyses have identified a central role for IgE/FcεRI cross linking on the surface on mast cells (MC) in promoting the clinical manifestations associated with food-triggered anaphylaxis10–16. MCs upon IgE cross linking release an array of preformed mediators including histamine, PAF, serotonin, proteases (tryptase and chymase), and lipid-derived mediators (prostaglandins [PGD2] and leukotrienes [LTC4, LTD4 and LTE4]) which are thought to drive the systemic and GI symptoms11,12,14,15,17. Genetic or pharmacological blockade of MC activity prevents the GI and systemic involvement in oral antigen-induced anaphylaxis18,19. Notably, experimental investigations have revealed that histamine via histamine receptor (HR) HR1, HR2 and HR4 signaling drives the systemic manifestations of IgE-mediated anaphylaxis in mice18,20,21, however the GI symptoms while MC and IgE-dependent, remain unabated by HR1 and HR2 antagonism18 indicating a role for other MC-derived mediators in the induction of the GI symptoms of oral antigen-induced anaphylaxis.
Herein, we describe the temporal molecular processes associated with GI symptoms of food-induced anaphylaxis in mice. We show that oral antigen-challenge induces a rapid dysregulation of small intestine (SI) transcellular Cftr-dependent Cl− secretion and paracellular macromolecular leak. The paracellular macromolecular flux was linked with modification of transmembrane proteins in the tight junction (claudin-1,2,3, 5 and junctional adhesion molecule-A) and epithelial cadherins in the adherens junction (E-cadherin) and desmosomes (DM) (Desmoglein 2 and desmocollin 2), and was mediated by IgE/MC activation. We show that GI manifestations such as secretory diarrhea was antigen-induced and persisted for 72 hours and was abated by abrogation of proteolytic activity and Cl− secretion. Collectively, these studies suggest that MC-driven proteolytic activity and Cl− secretory response is required for the development of secretory diarrhea response during a food-induced anaphylactic reaction in mice.
Results
Food antigen exposure is restricted to the small intestine (SI) during a food-induced anaphylactic reaction.
We have previously demonstrated that OVA-sensitized mice demonstrate systemic and gastrointestinal symptoms including diarrhea within 30 min of the 7th oral OVA challenge22,23. To ascertain the localization of dietary antigen in the GI tract during the onset of the symptoms of food-induced anaphylaxis, mice received o.g. of fluorescent-OVA and the transit of dietary antigen along the GI tract was monitored for 30 min (Fig. 1A). We show that dietary antigen was predominantly localized in the SI, in particularly the jejunum-ileum region, with the highest concentration localized to the proximal ileum region (Fig. 1B). We observe minimal evidence of dietary antigen in the caecum and colon from the ileocecal junction to distal colon. OVA-sensitized and challenged mice possess a heightened GI CD4+ Th2, ILC2 immune response in the SI, which can alter GI peristalsis24–28. To determine the localization of antigen in the GI compartment following IgE-MC activation independent of the Type-2 immune response we employed the passive-oral IgE mediated model of anaphylaxis using transgenic mice with intestinal mastocytosis and no Th2 activation (iIL-9Tg)19,22. Notably, fluorescent OVA in iIL-9Tg mice 30 min following MC activation was similar to that observed in WT mice that experienced food-induced anaphylaxis (Fig 1B). Furthermore, the dietary antigen was restricted to the distal jejunum and jejunoileal region in naïve WT mice and iIL-9Tg mice that received isotype control and did not experience anaphylaxis, suggesting that anaphylaxis does not significantly alter dietary antigen translocation (Fig 1B). These studies indicate that the eliciting dietary antigen is predominantly restricted to the murine jejunoileal region and not in the caecum or colon at the corresponding time these mice experience symptoms of anaphylaxis.
Fig. 1. Eliciting antigen is restricted to the SI during the onset of food-induced anaphylactic symptoms.
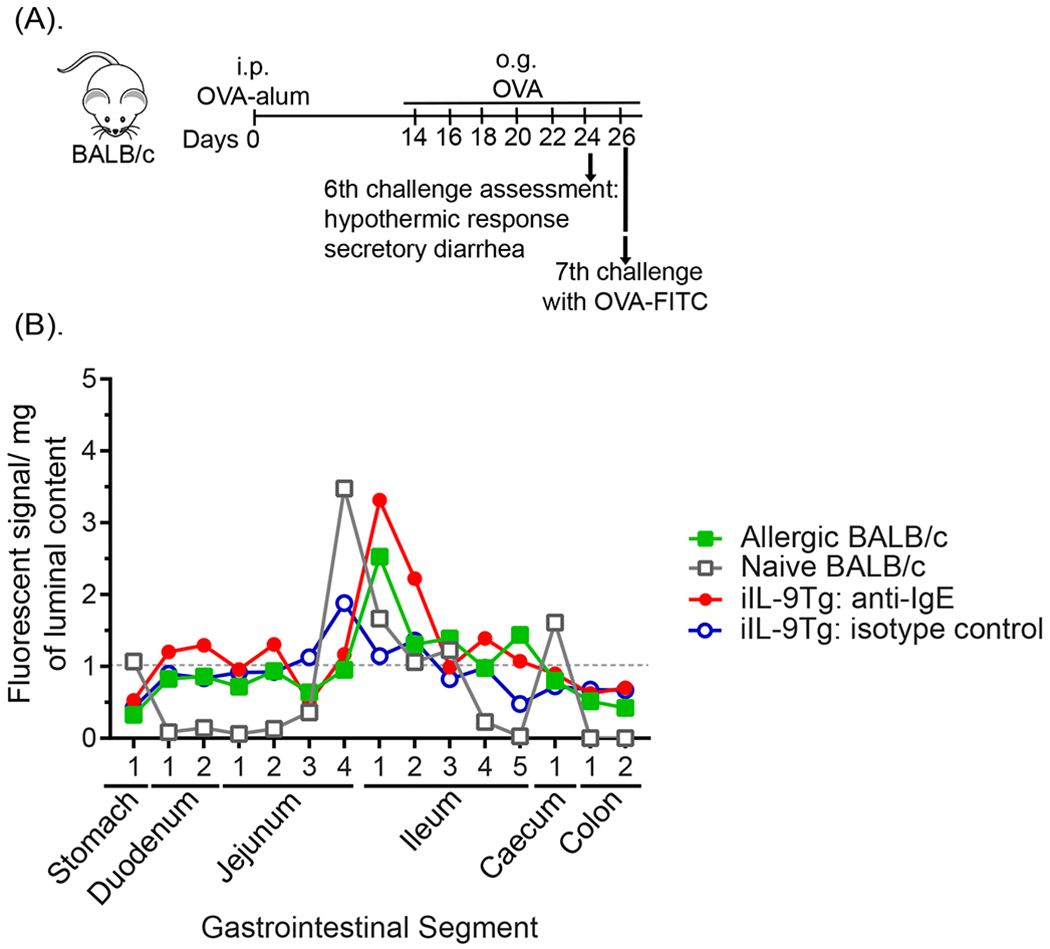
(A) Experimental regimen, (B) Localization of OVA-fluorescence in the GI tract segments of naïve or food allergic WT and iIL-9Tg mice following anti-IgE treatment. OVA-sensitized BALB/c mice were repeatedly challenged with OVA and on the 7th challenge received 5 x 105 FITC-labelled FluoSpheres™ Polystyrene Microspheres and localization of FluoSpheres in the GI segments were examined by fluorescence within 30 min. Naïve mice and iIL-9Tg mice challenged with either anti-IgE (20μg / 200μl i.v.) or vehicle received oral gavage of 5 x 105 FITC-labelled FluoSpheres™ Polystyrene Microspheres and localization of FluoSpheres in the GI segments were examined within 30 min. Data are represented as the mean fluorescence signal detected in luminal contents (per mg) of the respective GI segments from n = 5 mice.
Antigen challenge stimulated intestinal epithelial CFTR-dependent Cl− transport and paracellular leak.
Given our observation that dietary antigen was restricted to the SI, we examined epithelial ion transport (Short circuit current; Isc) of the SI from control and food-allergic mice (within 30 min of food-challenge) to determine whether anaphylaxis was associated with altered intestinal epithelial permeability. Basal Isc and forskolin-induced ΔIsc of the SI of food allergic mice was significantly increased compared to vehicle-treated mice (Fig. 2A and B). Pre-exposure of the SI epithelium to the CFTR inhibitor (CFTRinh172) and not DIDS, a potent inhibitor of calcium activated Cl− transporters (Cl-/HCO3− exchanger and potassium/chloride co-transporter), abrogated the Forskolin-induced ΔIsc indicating that increased current is predominantly mediated by CFTR-dependent Cl− transport activity (Fig. 2C). Assessment of the paracellular epithelial function of the jejunum from OVA-treated mice revealed decreased Transepithelial resistance (TER) and increased macromolecular flux (FITC-Dextran flux; apical to basolateral) compared with jejunal preparations from vehicle-treated mice (Fig. 2D and E). Collectively, these studies suggest that dietary antigen induced a rapid SI epithelial CFTR-dependent Cl− secretory response and paracellular permeability within 30 min of antigen challenge.
Fig. 2. Oral antigen challenge induced SI epithelial transcellular and paracellular barrier dysfunction.
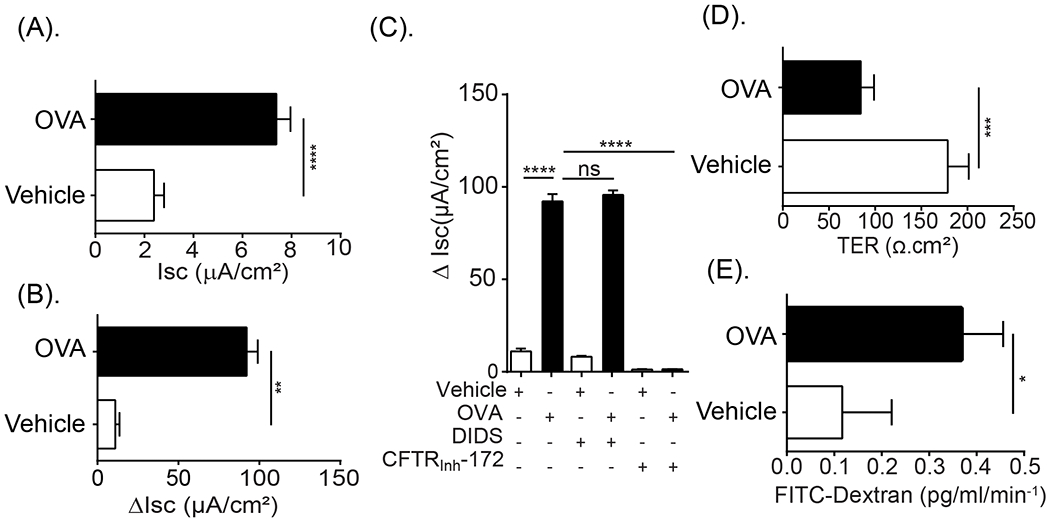
(A) Isc baseline, (B) Forskolin-induced short-circuit current response (ΔIsc) of jejunum segments from Vehicle- and OVA-treated BALB/c WT mice within 60 min of the 7th OVA challenge. (C) Forskolin-induced Short-circuit current response of jejunum segments from Vehicle- and OVA-treated mice following exposure to the ion channel blockers DIDS (100 μM) or CFTRInh172 (20 μM) in the mucosal reservoir inside Ussing chambers system. (D) TER and (E) FITC-dextran flux of jejunum segments from Vehicle- and OVA-treated BALB/c WT mice within 60 min of the 7th OVA challenge. OVA-treated mice were sensitized with OVA-alum and received seven o.g. OVA challenges. Vehicle-treated mice are OVA-sensitized mice that were challenged with vehicle (Saline) and did not develop anaphylaxis symptoms. Data are represented as the mean ± SD; n = 3 - 7 mice per group from 2 - 3 representative experiments (A-E). **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05, ns > 0.05.
Direct exposure of OVA to jejunal SI preparations is sufficient to promote epithelial transcellular and paracellular dysfunction.
Next, we examined whether the food-induced macromolecular leak was associated with changes in intercellular junctional proteins (JP). To do this, we examined SI epithelial AJ and TJ proteins from naïve mice and mice that demonstrated symptoms of anaphylaxis (≥ 1.5 °C Temperature loss and diarrhea) 30 min following the 7th food allergen challenge. We observed a significant reduction in the level of full length transmembrane proteins in the TJ, Claudin −1, −2, −3 and −5 AJ, E-cadherin and DM, Desmoglein-2 (Dsg-2) and Desmocollin-2 (Dsc-2) in SI epithelial extracts from food allergic mice compared with naïve mice (Fig 3A and B, control (CTL) vs. food-challenge #7). Intriguingly, we identified the presence of cleaved fragments of the AJ protein, E-cadherin (~55 kDa) and Dsg-2 (22 kDa) and Dsc-2 (75 kDa) in SI epithelial extracts from anaphylactic mice (Fig 3B, food-challenge #7). The food allergic reaction was not associated with a decrease in all intestinal JP as the SI TJ protein occludin was unaffected by repeated dietary food-challenge (Fig 3A challenge #7). Keratin-8 (CK-8) immunoblotting reveals comparable level of intestinal epithelial cells and Actin and GAPDH shows similar protein loading (Fig 3A and B). Immunofluorescence analyses of the jejunum revealed a similar pattern of decreased proteins in the TJ, Claudin-1, Claudin-2 and E-cadherin in the apical junctional complex of the intestinal epithelium of allergic mice within 30 min of antigen challenge as compared with control mice (Fig. 3D). Notably, we observed no change in the expression of the transmembrane protein ZO-1 in jejunum of allergic mice following allergen exposure.
Fig. 3. Oral antigen-induced SI para-cellular dysfunction is associated with degradation of adherence and tight junction proteins.
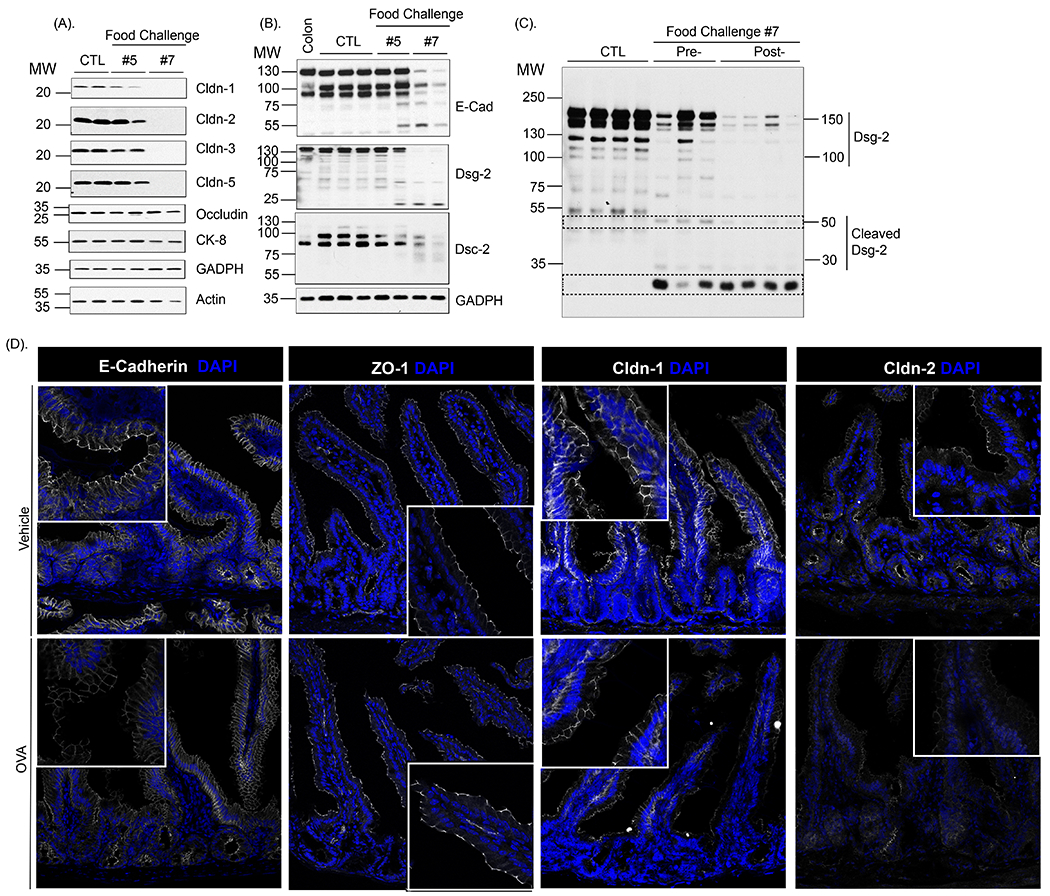
Assessment of intestinal junctional proteins expression: Western protein analysis of (A) Claudin-1, Claudin-2, Claudin-3, Claudin-5, Occludin, Keratin-8, GADPH and Actin and (B) E-cadherin, Dsg-2, Dsc-2 and GADPH in isolated jejunal epithelial cells from untreated (CTL) and OVA-sensitized mice 30 min following the 5th and 7th oral challenge. (C) Western protein analysis of Dsg-2 in isolated jejunal epithelial cells from untreated (CTL) and OVA-sensitized mice prior to (Pre-) and 30 min following the 7th oral challenge (Post-) (A-C). Actin and GAPDH were used as a loading control. Colonic tissue was used as a positive control. MW, Molecular weight. Each column represents a single mouse (D) Immunofluorescence analysis of E-cadherin, ZO-1, Claudin-1 and Claudin-2 (white) in jejunum segments from Vehicle- and OVA-treated BALB/c WT mice within 30 min of the 7th OVA challenge. Nuclei are visualized with DAPI (blue).; (D). Representative photomicrographs of n = 5 - 7 mice per group and 5 serial SI sections per mouse.
To determine whether the cleaved intestinal JP in the TJ, AJ and DM was associated with development of GI symptoms of food-induced anaphylaxis, we performed analyses on mice following the fifth food-challenge that do not demonstrate symptoms of food-induced anaphylaxis following challenge (post-5th challenge). We show that the level of TJ proteins, Claudin −1, −2, −3 and −5 were similar to that observed in naïve mice (Fig 3A; food-challenge #5). Furthermore, we observed full length E-cadherin and Dsg-2 and Dsc-2 in SI epithelial extracts from asymptomatic mice following 5th challenge (Fig 3B), albeit we detected the presence of cleaved cadherin fragments (E-cadherin and Dsg-2 and Dsc-2) (Fig 3B). Collectively these data suggest that cleavage of intestinal JP in the TJ, AJ and DM was associated with development of GI symptoms of food-induced anaphylaxis.
Given that we were able to detect cleaved cadherin fragments in the AJ and DM, we utilized the Dsg-2 western blot analyses as a surrogate marker to determine whether a single dietary antigen-challenge induced rapid SI cadherin cleavage. To do this, we examined SI epithelial Dsg-2 in mice prior to (Pre-) and following (Post-) the 7th food-challenge. Notably, we observed loss of the native full length Dsg-2 following the 7th challenge. Additionally, we also observed decreased levels of the 50 kDa Dsg-2 cleavage fragment and accumulation of lower molecular weight (30kDa) Dsg-2 fragment (Fig 3C). These data indicate that a single allergen challenge is sufficient to induce a pronounced and rapid decrease in the full length high molecular weight Dsg-2 protein levels and increasing low molecular weight Dsg-2 cleavage products in mice that develop food-induced anaphylaxis.
To determine whether direct antigen exposure of the SI epithelium can induce the GI epithelial dysfunction, we exposed the apical surface of SI segments from naïve and food-allergic mice ex vivo to OVA in an Ussing Chamber system and assessed Isc and TER. Ex vivo exposure of OVA to the SI segment of a naïve animal did not induce any significant change in Isc or TER (Fig. 4A). In contrast, OVA exposure of the SI segment from allergic mice stimulated an increase in Isc and a decrease in TER within 30 min (Fig. 4A and B). The baseline Isc and TER of the SI segments from allergic mice was trending lower than that observed from naïve mice, however levels were not statistically significant (Fig. 4A and B). These studies show that a single allergen challenge is sufficient to induce a pronounced and rapid decrease in intestinal epithelial barrier function that is related to enhanced ion transport and loss of intestinal epithelial barrier. To get insight into the temporal nature of the paracellular and transcellular epithelial dysfunction following allergen challenge, we monitored Isc and TER of SI segments from food allergic mice following the 7th OVA-challenge. We show that OVA-induced intestinal epithelial barrier dysfunction was maintained for at least 48 hours following dietary antigen challenge and returned to baseline levels by 72 hours (Fig. 4 C and D). Collectively, these studies demonstrate that dietary antigen exposure of the SI mucosal epithelium is sufficient to induce SI barrier dysfunction that can be sustained for up to 48 hrs following allergen exposure.
Fig. 4. Oral antigen exposure leads to temporal loss of epithelial transcellular and paracellular dysfunction.
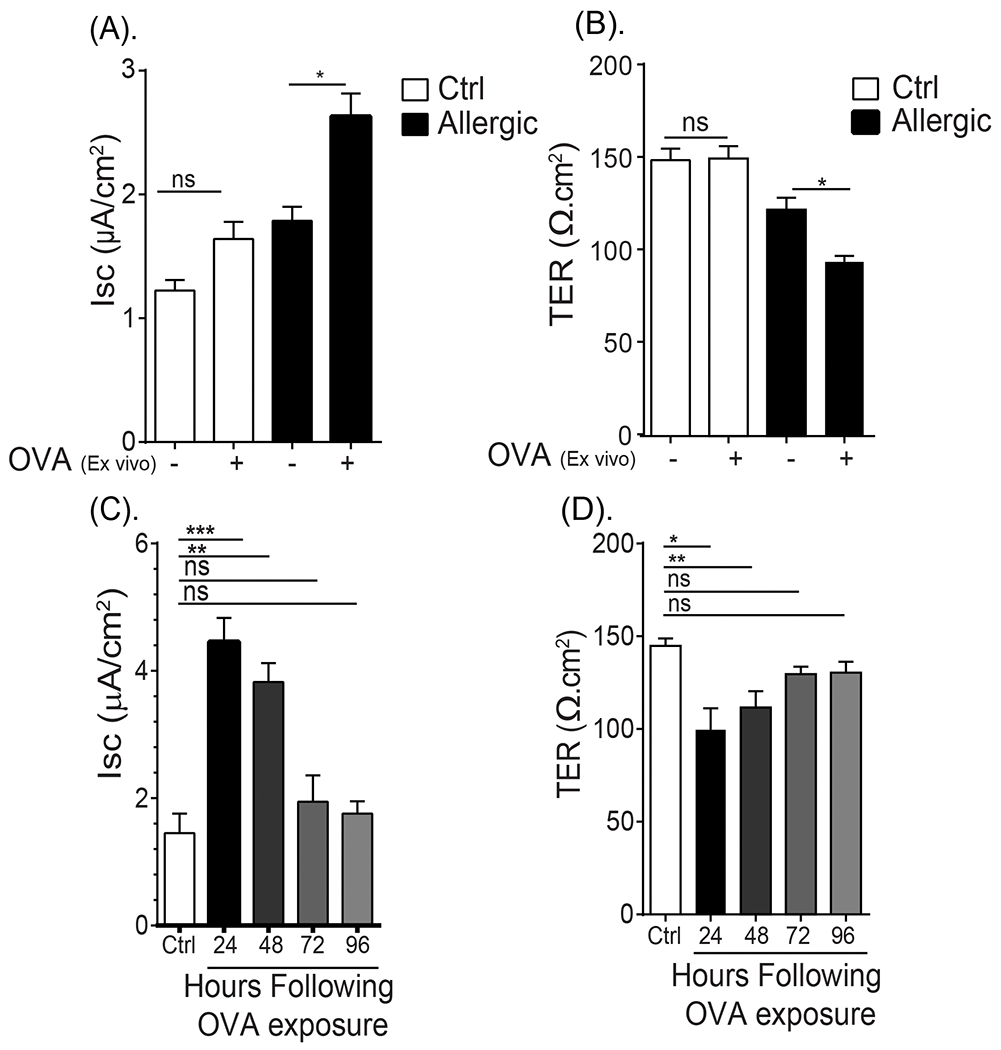
(A) Isc baseline and (B) TER of ex vivo jejunal segments from unsensitized (naïve) and OVA-sensitized and oral challenged mice (allergic) apically exposed to vehicle (PBS) or OVA in Ussing Chamber system. (C) Isc and (D) TER of ex vivo jejunal segments from unsensitized (Ctrl) and OVA-sensitized and oral challenged mice 24 - 96 hours following the seventh oral challenge. (A and B) Jejunum was removed from unsensitized (naïve) and OVA-sensitized and oral challenged mice (allergic- following 6th oral challenge) and mounted in a Ussing Chamber system and exposed to either PBS (−) or 1% OVA on the apical side and Isc and TER were recorded as described in the material and methods section. (C and D) Jejunum from unsensitized (Ctrl) and OVA-sensitized and oral challenged mice were removed 24, 48, 72 and 96 hours following seventh challenge and mounted in a Ussing Chamber system and Isc and TER were recorded as described in the material and methods section. Data are represented as the mean ± SD, n = 3 mice per group. **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05, ns > 0.05.
Dissection of mechanisms of oral antigen-induced transcellular and paracellular permeability.
To define the relationship between SI epithelial transcellular and paracellular barrier dysfunction and the development of the dietary antigen-induced GI symptom, secretory diarrhea, we examined the SI from allergic mice that did and did not develop diarrhea following the 7th challenge. The SI segment from mice that developed secretory diarrhea within 30 min of food allergen-challenge had a significant increase in Isc (~ 3-fold) and dramatically decreased TER (~60% reduction) compared to vehicle-treated mice (Fig. 5A and B). In contrary, the SI from mice that received antigen that failed to develop secretory diarrhea did not demonstrate evidence of altered Isc but did show a significant reduction in TER (~26% reduction) compared to vehicle-treated mice (Fig. 5A and B). These studies demonstrate a relationship between altered SI transcellular and paracellular permeability and the development of the food-induced symptom secretory diarrhea in food allergic mice.
Fig. 5. SI transcellular the paracellular permeability was associated with the development of oral antigen-induced secretory diarrhea.
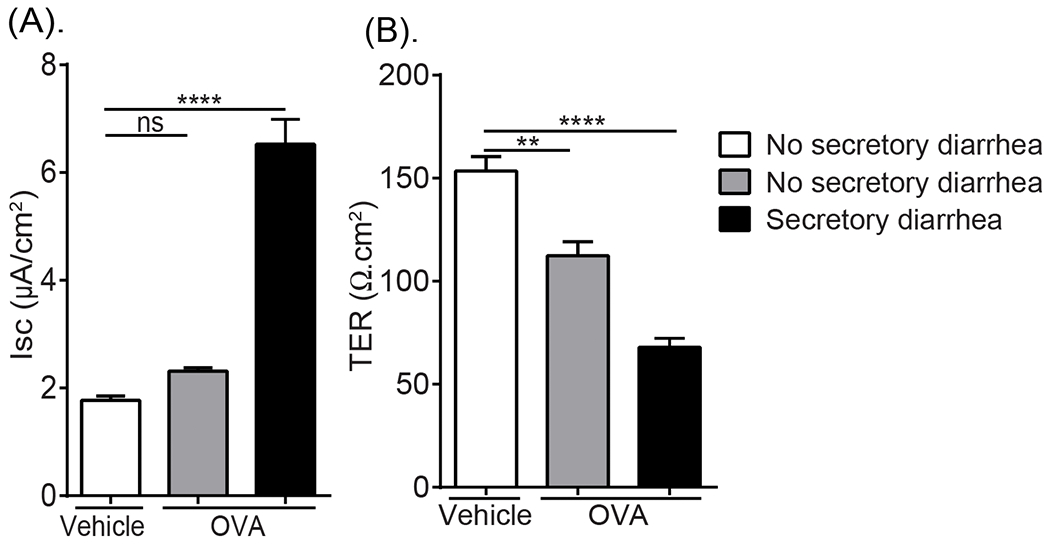
(A) Isc baseline and (B) TER measurements of jejunal segments from OVA-sensitized mice following Vehicle (Saline) or OVA oral challenge (7th challenge) and stratified according to secretory diarrhea development (60 min). OVA-sensitized mice were challenged with OVA (7th Challenge) and evidence of profuse liquid stool (diarrhea-positive) was examined. Following the 60 min diarrhea observation period, jejunal segments were removed and mounted in a Ussing Chamber system and Isc and TER were recorded as described in the Material and Methods section. Data are represented as the mean ± SD, n= 4 - 7 mice per group. **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05, ns > 0.05.
Given our demonstration of dietary antigen-induced enhanced Cl− transport, and also paracellular barrier dysfunction associated with JP protein degradation and that this was associated with the GI symptom diarrhea, we hypothesized that the secretory diarrhea response was a consequence of CFTR-dependent Cl− secretion and proteolytic-activity. To test this hypothesis, mice that demonstrated a history of food-induced anaphylaxis (as confirmed by the 6th challenge) received either a chloride channel blocker (GlyH101) (o.g 15 min before OVA) or a protease inhibitor (AEBSF) (i.v 2h before OVA) alone or in combination prior to the 7th challenge and food allergen–induced SI epithelial transcellular and paracellular function was assessed (Fig. 6A). As previously demonstrated, OVA-challenge of OVA-sensitized mice increased SI basal Isc, forskolin-induced ΔIsc response and FITC-dextran flux and reduced the TER compared to non-allergic (Vehicle) mice (Fig. 2 and Fig. 6B and C). Pretreatment with GlyH101 alone, significantly attenuated OVA-induced amplification of forskolin-induced ΔIsc, TER and FITC-dextran flux (Fig. 6B–E). Pretreatment with AEBSF alone, also significantly attenuated OVA-mediated induction of forskolin-induced ΔIsc, TER and FITC-dextran flux, however SI forskolin-induced ΔIsc was unaffected (Fig. 6B-E). Pretreatment with both GlyH101 and AEBS prior to the 7th OVA-challenge significantly attenuated OVA-induced dysregulation of both SI epithelial transcellular and paracellular function compared with OVA-treated mice that received vehicle (Fig. 6 B–C). Importantly, pretreatment of mice with GlyH101 and AEBSF dramatically reduced the incidence of diarrhea in mice following the 7th OVA-challenge (Fig. 7). Moreover, 10 of 10 of food allergic mice that received OVA developed secretory diarrhea following the 7th challenge. In contrast, only 8 of 18 mice who received GlyH101 and AEBSF developed secretory diarrhea following the 7th challenge (Fig. 7). Collectively, the GI symptom of dietary antigen-induced anaphylaxis, secretory diarrhea, is a consequence of food antigen-induced transcellular and paracellular SI epithelial barrier function.
Fig. 6. Inhibition of Cl− secretory activity and proteolysis attenuated oral antigen-induced SI transcellular the paracellular permeability.
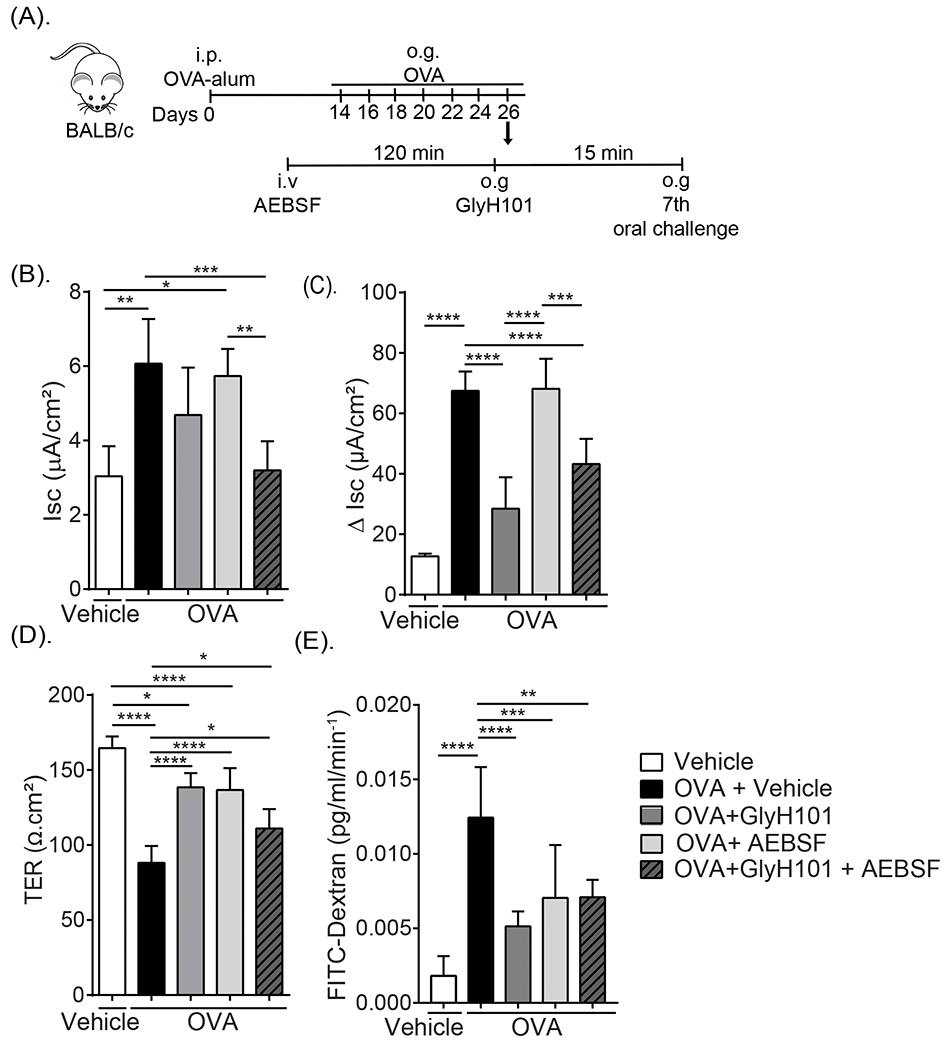
(A) Experimental regimen, (B) Isc baseline and (C) Forskolin-induced Isc responses (ΔIsc), (D) TER, (E) FITC-dextran flux of jejunal segments from OVA-sensitized and oral challenged mice (7th Challenge) following pretreatment with GlyH101 and protease inhibitor (AEBSF) alone or in combination. OVA-sensitized mice received repeated OVA challenge (six challenges) and mice that demonstrated evidence of food allergy were stratified into indicated groups. Mice received either 0.5 mM GlyH101 (oral gavage) 15 min prior to the 7th OVA-challenge or 500 μg AEBSF (i.v.) 2 hours prior to 7th OVA-challenge, alone or in combination and subsequently received oral gavage (OVA). Following the 60 minute observational period, jejunal segments were removed and mounted in a Ussing Chamber system and physiological measurements were recorded as described in the Material and Methods section. Vehicle represents unsensitized mice that received Vehicle oral gavage challenge. (C-E). Data are represented as the mean ± SD, n= 3 - 7 mice per group. **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05, ns > 0.05.
Fig. 7. Inhibition of Cl− secretory activity and proteolysis was associated with inhibition of GI symptoms of food-induced anaphylaxis.
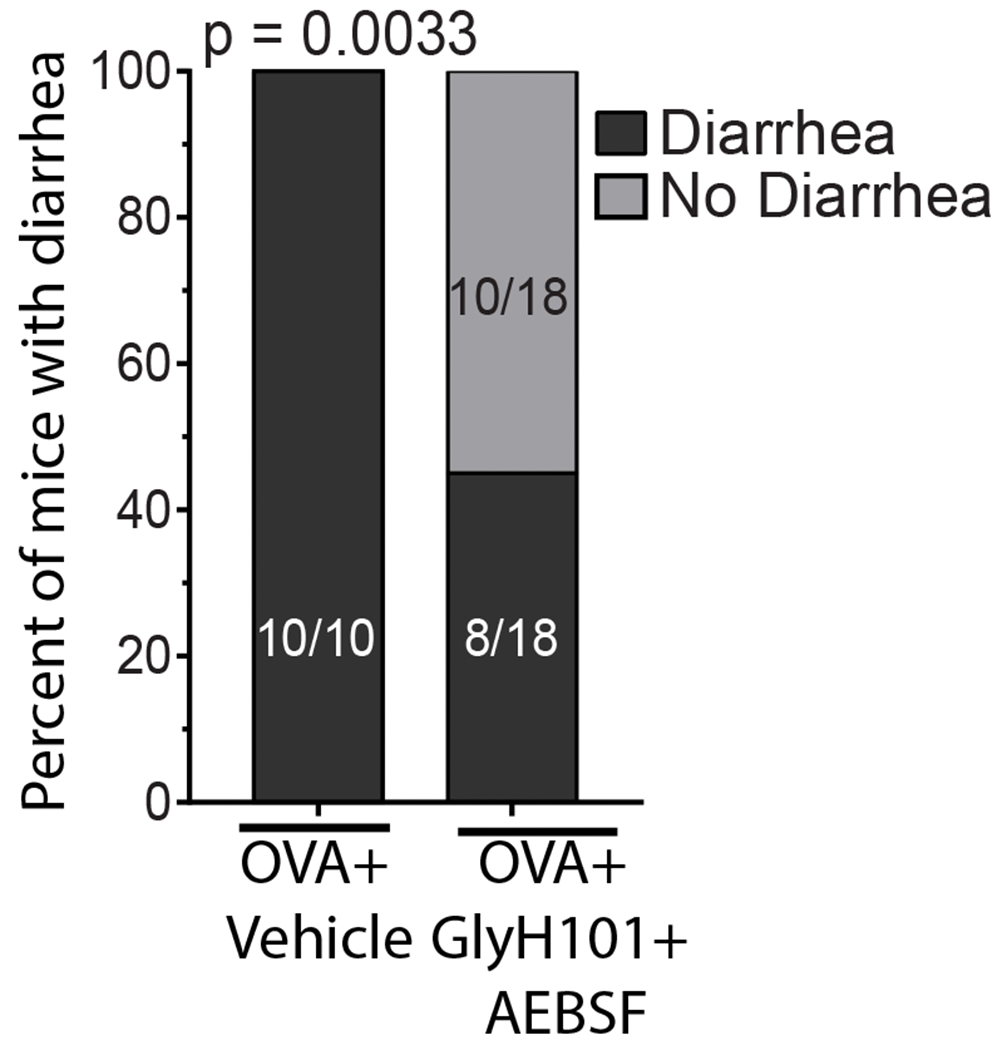
Chi-square analysis of number of mice with/without secretory diarrhea. OVA-sensitized mice received repeated OVA challenge (six challenges) and mice that demonstrated evidence of food allergy were stratified into either Vehicle or GlyH101 + AEBSF groups. Mice received 500 μg AEBSF (i.v.) 2 hours prior to 7th OVA-challenge and 0.5 mM GlyH101 (oral gavage) 15 min prior to the 7th OVA-challenge and subsequently received oral gavage (OVA) and evidence for diarrhea was examined for a 60 min observational period.
Discussion
In the present study, we show that: 1) the onset of GI symptoms of dietary antigen-induced anaphylaxis occurred with dietary antigen localized to the SI; 2) the symptom of diarrhea was associated with increased SI epithelial CFTR-dependent Cl− secretion and epithelial JP degradation; 3) The transcellular and paracellular SI dysfunction occurred rapidly following food antigen exposure and persisted for up to 72 hours; 4) development of the intestinal symptom diarrhea in dietary antigen-induced anaphylaxis was linked to SI transcellular and paracellular dysfunction and 5) blockade of chloride channel and proteolytic activity attenuated the GI symptoms in dietary antigen-induced anaphylaxis.
The association between food allergy and diarrhea dates back to the 1930s29–31. Rowe reported cases of diarrhea in 15% of 150 cases of GI allergy. Whether or not the symptom of diarrhea was associated with and acute-IgE-mediated reaction or part of some other allergic condition (e.g. allergic colitis or FPIES) was inconclusive, however the diarrhea phenotype was food-dependent, acute and often associated with other clinical signs and symptoms of food-induced anaphylaxis such as fatigue, toxemia and nervousness29,30. More recent reports indicate that IgE-mediated anaphylactic reactions involving the GI tract including diarrhea occur in 30 to 45% of cases anaphylaxis32,33. Interestingly, antidotal data supports a role for GI symptoms in the severity of food induced anaphylaxis4,9. Retrospective chart analyses of clinical features of acute generalized hypersensitivity reactions in 1,149 patients revealed that features of anaphylaxis, including vomiting and nausea, strongly correlated with anaphylaxis severity34. In contrast, common skin features, including urticaria, erythema and angioedema, did not correlate with hypoxia and hypotension34. Similarly, examination of the clinical history of 163 pediatric patients with food-induced anaphylaxis showed that relapsing GI symptoms increased the risk of hypotension and bradycardia or cardiac arrest8.
The molecular basis by which an eliciting dietary antigen induces GI symptoms in food-induced anaphylaxis is unclear. We have previously shown that oral antigen-induced anaphylaxis in mice is MC-dependent18 and that heightened GI MC numbers correlate with intestinal and systemic anaphylaxis severity19,22. Similarly, systemic mastocytosis patients can present with increased GI symptoms and have increased risk of severe anaphylaxis35,36 and administration of the MC stabilizing agent sodium cromoglycate protected food allergic individuals from food provocation-induced GI permeability and altered GI function37. Herein, we demonstrate that the food allergen-induced GI symptom of diarrhea was associated with a rapid SI secretory response and epithelial barrier dysfunction and that suppression of the GI symptom required inhibition of both Cl− channel activity and proteolysis. Several MC-derived mediators are known to modulate discrete components of the intestinal epithelial secretory and barrier function including histamine, prostaglandins and mast cell-derived proteases38,39. Secretory diarrhea is generally a result of dysregulation of the coordinated GI epithelial secretory or absorptive processes leading to excessive accumulation of GI luminal fluid40. The SI epithelial fluid secretion is predominantly mediated by transepithelial Cl− flux from the basolateral to apical surface of the epithelium via coordinated chloride channels and transporters including Na/K/Cl symporter (NKCC1), Basolateral K+ channels (KCNQ1/KNE3 and KCNN4), the cyclic-nucleotide-activated cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channels (CaCCs)41. We show that dietary exposure of the SI of food-sensitized mice promoted an increase in basolateral to apical Cl− secretion via CFTR-dependent mechanism. Histamine signaling through the H1R stimulates CFTR Cl− ion secretion through cAMP/PKA pathway42,43. Similarly, prostaglandins and serotonin increase Cl− secretion in human colon and jejunum samples respectively44,45. However, experimental studies in in vivo animal model systems have demonstrated that while antihistamines may impact systemic symptoms, they do not impact the secretory diarrhea phenotype in food-induced anaphylaxis, and clinically antihistamines have been shown to be effective for the treatment of the cutaneous symptoms but not the gastrointestinal symptoms3,18. A recent study reported an important role for MC-derived PGD2 in the suppression of systemic symptom of shock (hypotension and hypothermia) during an anaphylactic reaction in mice46; however as the anaphylaxis model employed by the investigators did not induce GI symptoms, they were unable to assess the contribution of MC-derived PGD2 to the secretory diarrhea response. Mast cell-derived cytokines such as IL-6, IL-8, IL-13 and TNF alpha have been shown to activate secondary messenger cascades including Ca2+ and cyclic nucleotides such as cAMP and cGMP to stimulate CFTR dependent Cl− transport and also inhibition of the absorptive capacity of apical Na+ transporters and promote a secretory diarrhea phenotype40,47. Furthermore, the pro-Type 2 cytokines associated with food-induced anaphylaxis such as IL-4 and IL-13 regulate SI CFTR expression and enhanced CFTR activity47–49. However, IgE-FcεRI-induced release of cytokines from MCs generally occurs up to 1 hour following activation and we show that the dietary antigen-induced CFTR-dependent response occurred rapidly within min of allergen exposure suggesting that the CFTR dependent Cl− transport is not likely MC-derived cytokine-mediated. We predict that the repetitive oral allergen challenge and stimulation of the SI CD4+ Th2 and ILC2 response is likely to drive SI CFTR mRNA and protein induction in the intestinal epithelium and that cAMP-inducing mast cell-derived mediators stimulate exaggerated intestinal epithelial CFTR activity and CFTR-dependent Cl− secretion and as a consequence development of secretory diarrhea acutely following allergen exposure. The physiological significance of increased fluid secretion in the SI of food allergic mice is likely related to efforts to remove the food allergen from the SI surface epithelium and promote elimination of the food allergen from the host GI compartment. Analogous to this, helminth infestation of the GI tract is known to promote an anti-helminth host immune response known as the “weep and sweep”. The “weep and sweep” response involves induction of a GI CFTR-dependent secretory response to increased luminal fluid (weep) and increased peristaltic contractility (sweep) which is thought to lead to detachment of the helminth parasite from the surface epithelia and promote parasite expulsion from the host50. Intriguingly, the anti-helminth host “weep and sweep” response is driven by a CD4+ Th2 and ILC2-dependent immune response similar to that observed in food allergic reactions50,51.
The dietary antigen-induced SI epithelial barrier dysfunction was also associated with rapid degradation in the SI epithelial TJ and AJ proteins and increased paracellular leak. AJ and TJ proteins expressed by intestinal epithelial cells are critical of the establishment and maintenance of intestinal epithelial paracellular permeability and barrier function52,53. Previous studies in rats have revealed increased intestinal permeability during an intestinal hypersensitivity reaction that was dependent on MC activation and release of MC proteases54–56. IgE-crosslinking of FcεRI on MC promotes the rapid release of several proteases in both mouse and man57–60. In mice, IgE-mediated reactions are associated with the prodigious release of the chymotrypsin-like serine protease MCPT-1, however connective and mucosal MCs are known to express many additional proteases including MCPT-4 (chymotrypsin-like), MCPT-5 (Elastase-like), MCPT-6 and MCPT-7 (trypsin-like tryptases) and carboxypeptidase 3 (CPA3)61. Several of the MC proteases can disrupt TJ proteins and increase cellular permeability. MCPT-4, which possesses chymotryptic proteolytic activity, and the elastase-like MCPT-5 have been shown to disrupt epidermal TJ function, in particularly Claudin-462. Studies in MCPT-1-deficient mice have revealed a role for MCPT-1 in the proteolytic degradation of Occludin63 and MCPT-4 induces disruption of SI epithelial Claudin-3 function via PAR-2-dependent process and altering SI intestinal permeability64,65. Similarly, human tryptase has been shown to regulate GI permeability via PAR-2-β-arrestin-dependent mechanism causing disruption of TJ, Claudin-1, ZO-1 and Occludin and perijunctional F-actin66,67. Food-induced anaphylaxis in mice is associated with the accumulation and degranulation of intestinal mucosal MCs, and not connective tissue MCs, suggesting that mucosal MCs proteases such as MCPT-1 and MCPT-2 may drive the SI epithelial barrier dysfunction18,19. Employing a rat model of anaphylaxis, investigators have previously reported a role for the mucosal mast cell granule chymase, rat mast cell protease-II (RMCP-II), in increased jejunal paracellular permeability56,68. Notably, RMCP-II induces loss of ZO-1 and Occludin in MDCK-II monolayers56,68. Examination of Mcpt1−/− mice on the BALB/c background, revealed no role for MCPT-1 in the incidence of diarrhea or severity of food-induced anaphylaxis (Incidence of Diarrhea; 8 / 10 vs 9 / 14; following the 7th oral (OVA) challenge of OVA-sensitized WT vs Mcpt1−/− mice; n = 10 and 14 mice, respectively.) suggesting that other mucosal MC-derived proteases are likely to drive the SI epithelial barrier dysfunction.
In summary, by employing a murine model of dietary antigen-induced anaphylaxis with intestinal and systemic symptoms, we show that the dietary antigen-induced anaphylaxis is associated with an increase in SI transcellular and paracellular permeability. We show that the altered intestinal permeability and secretory diarrheal phenotype is rapidly induced by MC degranulation and can be inhibited by pharmacological blockade of proteolytic and CFTR-dependent Cl− transport activity.
Materials and Methods
Animals.
6-8-week-old BALB/c wild type (WT) mice were obtained from the National Cancer Institute (Bethesda, MD, USA) and bred in-house at Cincinnati Children’s Hospital Medical Center (CCHMC) (Cincinnati, OH, USA) and at the University of Michigan (UM) (Ann Arbor, MI, USA). Intestinal IL-9 transgenic (iIL9Tg) mice were generated as previously described19. Age-, sex-, weight-matched littermates were used as controls in all experiments. The mice were maintained and bred in a clean barrier facility and were handled under an approved Institutional Animal Care and Use Committee protocols at CCHMC and University of Michigan animal facility.
Oral antigen-induced intestinal anaphylaxis.
4-8-week-old mice were sensitized to ovalbumin (OVA) (50 μg of OVA / 1 mg of alum in sterile saline by intraperitoneal (i.p.) injection) and received repeated oral gavage (o.g.) challenge with OVA (250 μl of OVA (50 mg) in saline or 250 μl of saline (vehicle)) as previously described22. Prior to each o.g. challenge, mice were deprived of food for 4-5 h. Rectal temperatures were measured prior to challenge and then every 15 min for 60 min. Diarrhea was assessed by visually monitoring mice for up to 60 min following o.g. challenge and mice demonstrating profuse liquid stool were recorded as diarrhea-positive. Evidence of secretory diarrhea was assessed by determination of short-circuit current (Isc) of SI segments ex vivo in a Ussing chamber system up to 60 min following o.g. challenge. Mice were considered allergic if they demonstrated symptoms of anaphylaxis (hypothermia > 1.5 °C Temperature loss and diarrhea) following the 6th or 7th challenge. In Some experiments, mice were o.g. with 0.5 mM N-(2-naphthalenyl)-[(3,5-dibromo-2,4-dihydroxyphenyl) methylene] glycine hydrazide (GlyH101) (EMD Millipore #219671) 15 min before the 7th OVA-challenge. 500 μg 4-benzenesulfonyl fluoride hydrochloride (AEBSF) (Sigma# A8456) were giving intravenous (i.v.) 2 h prior to the 7th OVA the challenge. To track food allergen passage in the GI tract, Mice were administered OVA (200 mg/ml) with 5 x 105 FITC-labelled FluoSpheres™ Polystyrene Microspheres (10 μM size) (Thermo Fisher, Waltham, MA, USA) by oral gavage and monitored for 30 min. The mice were euthanized, the GI tract surgically removed and segmented into anatomical compartments of the GI tract (stomach, duodenum, jejunum, ileum, caecum and colon). The duodenum was divided into 1.5 cm segments, jejunum into 4 cm segments, ileum into 2 cm segments and colon into 4 cm segments. The duodenum was defined as 3 cm GI segment distal to pyloric sphincter. The jejunum was defined as the ~16 cm GI segment distal of the duodenum and 10 cm proximal from the ileocecal valve. The ileum was defined as the GI segment 10 cm proximal from the ileocecal valve. The caecum was defined as the pouch connecting to the junction of the proximal ileum and distal colon. The colon segment was ~ 8 cm connecting the proximal caecum to the distal rectum. The luminal contents of the segments were flushed with Phosphate-buffered saline (PBS) centrifuged and suspended in 200 μl PBS and the fluorescence of the total contents of each segment was measured using a Bioteck multi-mode plater reader (Synergy H1) with Gen5 software.
Passive Anaphylaxis.
Mice were injected i.v. with 20 μg / 200 μL of anti-IgE (IgG2a mAb to mouse IgE; EM-95) and evidence of anaphylaxis was examined as previously described69,11.
Solutions and drugs.
The Krebs buffer used on each side of the Ussing chamber contained 4.70 mM KCl, 2.52 mM CaCl2, 118.5 mM NaCl, 1.18 mM NaH2PO4, 1.64 mM MgSO4 and 24.88 mM NaHCO3. The tissues were allowed to equilibrate for 15 min in Krebs buffer containing 5.5 mM glucose. All reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise.
Ussing chambers.
1 cm, freshly isolated, serosal-stripped segments of jejunum were mounted between the hemi-chambers of an Ussing apparatus (U2500 Dual Ussing chamber, Warner instruments, Hamden, CT), and 0.112 cm2 of tissue was exposed to 10 ml Krebs buffer at 37 °C. The transepithelial potential difference (PD) was detected with two paired electrodes that contained 4% agar in 3 M KCl. The electrodes were connected to a voltage clamp amplifier (EC-800, Epithelial voltage clamp, Warner Instruments, Hamden, CT). The electrode potential difference and fluid resistance were compensated before mounting tissue segments into the chamber. To establish equilibrium, PD was continuously monitored under open-circuit conditions for 15 min. Thereafter, the tissues were voltage-clamped at 0 mV while continuously measuring Short circuit current (Isc). Voltage pulses (3-mV square waves sustained for 5 seconds) were delivered every 50 seconds to yield a current response for calculation of transepithelial resistance (TER) from Ohm’s law. For ion conductance experiments, changes in Isc were determined for the cumulative addition of forskolin and acetylcholine to the serosal reservoir. After the peak response to the final concentration of each agonist was recorded, the Krebs buffer on each side of the chamber was replaced, and the tissue was allowed to equilibrate for 30 min. Immediately following re-equilibration, tissue was pre-incubated with ion channel blockers 4,4’-Diisothiocyanatostilbene-2,2’-disulfonate (DIDS) (100 μM) or CFTRInh172 (20 μM) to mucosal reservoir. Changes in Isc were measured in response to the addition of forskolin to the mucosal side. To study effects of direct allergen application, 1% OVA or equal volume of PBS was directly added into apical side of the dissected jejunum mounted in the Ussing Chamber and Isc, TER were recorded as previously described19.
Intestinal epithelial cells (IEC) preparation.
5 cm segment of the jejunum was washed with cold PBS and 2% fetal bovine serum (FBS) and 5 mM DTT (20 min at 37°C with shaking). Afterward, IEC were isolated by washing tissue 3 times with PBS and 2% FBS and 5 mM EDTA (10 min at 37°C with shaking), then the washing solution was collected then centrifuged (400g for 10 min at 4°C) and pellet was suspended in PBS for cells quantification and lysis. For cell lysis, isolated IEC were resuspended in RIPA buffer (0.5% Triton X-100, 0.5% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 150 mM NaCl, 1 mM EGTA [pH 8.0], 1 mM EDTA, 0.2 mM sodium orthovanadate, 20 mM Tris [pH 7.4]) supplemented with protease and phosphatase inhibitors. Immunoblotting was performed as previously described70 .
Immunofluorescence.
5 cm segment of the jejunum was fresh frozen in O.C.T. Tissues were fixed in 95% cold ethanol for 30 min, followed by 1 min of pure acetone fixation at room temperature. For ZO-1 staining, tissues were fixed in 4% PFA, followed by permeabilization with 0.5% Triton X-100. Primary antibody staining was performed in Hank’s balanced salt solution with 3% bovine serum albumin (BSA) for overnight. Secondary antibodies were incubated in 3% BSA and for 1h. Antibodies for WB were as follows: Rabbit anti-claudin-1 #51-9100 (Thermo Fisher, Waltham, MA, USA), rabbit anti-claudin-2 #51-6100 (Thermo Fisher, Waltham, MA, USA), rabbit anti-claudin-3 #SAB4500434 (Sigma Aldrich, St. Louis, MO, USA), mouse anti-claudin-5 #35-2500, rabbit anti-ZO-1 #617300 (ThermoFisher, Waltham, MA, USA), goat anti-E-Cadherin #AF748, goat anti-mouse JAM-A #AF1077 (R&D Systems, Minneapolis, MN, USA), rabbit anti-cytokeratin-8 #ab53280, rabbit anti-desmoglein-2 #ab124683 (Abcam, Cambridge, United Kingdom), mouse anti-desmocollin-2 #32-6200 (Thermo Fisher, Waltham, MA, USA), rabbit anti-GADPH #G9545 (Sigma Aldrich, St. Louis, MO, USA), rabbit anti-calnexin #C4731 (Sigma Aldrich, St. Louis, MO, USA). Antibodies for immunofluorescence were as following: rat anti-E- cadherin #53-3249-82, rabbit anti-claudin-1 #51-9000, rabbit anti-claudin-2 #516100 (Thermo Fisher, Waltham, MA, USA). Nucleus were detected with DAPI. Confocal microscopy was performed using a Leica SP5 inverted microscope (Wetzlar, Germany) Leica SP5 software.
Statistical analysis.
Data are expressed as mean ± standard deviation (SD), unless otherwise stated. Statistical significance comparing different sets of mice was determined by Student’s t test. In experiments comparing multiple experimental groups, statistical differences between groups were analyzed using the one-way, nonparametric ANOVA and a Bonferroni post-test. P < 0.05 was considered significant. All analyses were performed using Prism 7.0 software (GraphPad Software Inc., San Diego, CA, USA).
Acknowledgment
This work was supported by National Institutes of Health grants DK073553, DK090119, AI138177, and AI112626; Food Allergy Research & Education (FARE); Department of Defense grant W81XWH-15-1-051730; M-FARA; and the Mary H. Weiser Food Allergy Center supported (to S.P.H.).
Abbreviations:
- IgE
Immunoglobulin E
- MC
Mast cells
- SI
Small Intestine
- OVA
ovalbumin
- Isc
Short circuit current
- TER
transepithelial resistance
- TJ
Tight junction
- AJ
Adherent junction
- DM
Desmosomes
- CFTR
Cystic Fibrosis transepithelial regulator
- GI
Gastrointestinal
- JP
Junctional protein
Footnotes
Disclosure
Authors have nothing to disclose
Bibliography
- 1.Sampson HA Anaphylaxis and emergency treatment. Pediatrics 111, 1601–1608 (2003). [PubMed] [Google Scholar]
- 2.Ross MP et al. Analysis of food-allergic and anaphylactic events in the national electronic injury surveillance system. J. Allergy Clin. Immunol 121, 166–171 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Wang J & Sampson HA Food anaphylaxis. Clin. Exp. Allergy 37, 651–660 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA, Mendelson L & Rosen JP Fatal and near-fatal anaphylactic reactions to food in children and adolescents. The New England journal of medicine 327, 380–384 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Schrander JJ, Unsalan-Hooyan RW, Forget PP & Jansen J [51Cr]EDTA intestinal permeability in children with cow’s milk tolerance. J. pediatr. Gastroenterol. Nutr 10, 189–192 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Troncone R, Caputo N, Florio G & Finelli E Increased intestinal sugar permeability after challenge in children with cow’s milk allergy or intolerance. Allergy 49, 142–146 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Van Elburg R, Heymans HS & De MJ Effect of disodiumcromoglycate on intestinal permeability changes and clinical response during cow’s milk challenge. Source (Bibliographic Citation): Pediatr Allergy Immunol 4, 79–85 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Calvani M. et al. Risk factors for severe pediatric food anaphylaxis in Italy. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 22, 813–819 LID - 8 10.1111/j.1399-3038.2011.01200.x [doi] (2011). [DOI] [PubMed] [Google Scholar]
- 9.Brown SGA Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 114, 371–376 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol 23, 749–786 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Strait RT, Morris SC, Yang M, Qu XW & Finkelman FD Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol 109, 658–668. (2002). [DOI] [PubMed] [Google Scholar]
- 12.Dombrowicz D, Flamand V, Brigman KK, Koller BH & Kinet JP Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell 75, 969–976 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Lorentz A, Schwengberg S, Mierke C, Manns MP & Bischoff SC Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol 29, 1496–1503 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Santos J, Benjamin M, Yang PC, Prior T & Perdue MH Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol 278, G847–854 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Kelefiotis D & Vakirtzi-Lemonias C In vivo responses of mouse blood cells to platelet-activating factor (PAF): role of the mediators of anaphylaxis. Agents and actions 40, 150–156 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Strait RT, Morris SC, Smiley K, Urban JF Jr. & Finkelman FD IL-4 exacerbates anaphylaxis. J Immunol 170, 3835–3842 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Lorentz A, Schwengberg S, Sellge G, Manns MP & Bischoff SC Human intestinal mast cells are capable of producing differnt cytokine profiles: Role of igE receptor cross-linking and IL-4. J. Immunol 164, 43–48 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Brandt EB et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 112, 1666–1677 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes EE et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med 205, 897–913 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsler JB, Schroeder HA, Byrne AJ, Chien KB & Bryce PJ Anaphylactic responses to histamine in mice utilize both histamine receptors 1 and 2. Allergy 68, 1338–1340, doi: 10.1111/all.12227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M. et al. Combined blockade of the histamine H1 and H4 receptor suppresses peanut-induced intestinal anaphylaxis by regulating dendritic cell function. Allergy 71, 1561–1574, doi: 10.1111/all.12904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahrens R et al. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. The American journal of pathology 180, 1535–1546, doi: 10.1016/j.ajpath.2011.12.036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sledd J et al. Loss of IL-4Ralpha-mediated PI3K signaling accelerates the progression of IgE/mast cell-mediated reactions. Immunity, inflammation and disease 3, 420–430, doi: 10.1002/iid3.80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiho H, Blennerhassett P, Deng Y & Collins SM Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 282, G226–232 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Akiho H, Ihara E, Motomura Y & Nakamura K Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World journal of gastrointestinal pathophysiology 2, 72–81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiho H et al. Interleukin-4- and −13-induced hypercontractility of human intestinal muscle cells-implication for motility changes in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol 288, G609–615, doi:00273.2004 [pii] 10.1152/ajpgi.00273.2004 [doi] (2005). [DOI] [PubMed] [Google Scholar]
- 27.Khan WI et al. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun 71, 2430–2438 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao A et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol 171, 948–954 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Rowe A Jr., Rowe AH, Uyeyama K & Young EJ Diarrhea caused by food allergy. The Journal of allergy 27, 424–436 (1956). [DOI] [PubMed] [Google Scholar]
- 30.Rowe AH [Food allergy and its clinical manifestations]. Revista clinica espanola 62, 366–373 (1956). [PubMed] [Google Scholar]
- 31.Rowe AH ABDOMINAL FOOD ALLERGY: ITS HISTORY, SYMPTOMATOLOGY, DIAGNOSIS AND TREATMENT. California and western medicine 29, 317–322 (1928). [PMC free article] [PubMed] [Google Scholar]
- 32.Simons FE & Sheikh A Anaphylaxis: the acute episode and beyond. BMJ (Clinical research ed.) 346, f602, doi: 10.1136/bmj.f602 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Lieberman P et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 126, 477–480 e471-442, doi: 10.1016/j.jaci.2010.06.022 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Brown SG Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 114, 371–376, doi: 10.1016/j.jaci.2004.04.029 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Soter NA, Austen KF & Wasserman SI Oral disodium cromoglycate in the treatment of systemic mastocytosis. The New England journal of medicine 301, 465–469, doi: 10.1056/nejm197908303010903 (1979). [DOI] [PubMed] [Google Scholar]
- 36.Horan RF, Sheffer AL & Austen KF Cromolyn sodium in the management of systemic mastocytosis. J Allergy Clin Immunol 85, 852–855 (1990). [DOI] [PubMed] [Google Scholar]
- 37.Andre C, Andre F, Colin L & Cavagna S Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of disodium cromoglycate. Annals of allergy 59, 127–130 (1987). [PubMed] [Google Scholar]
- 38.Perdue MH, Masson S, Wershil BK & Galli SJ Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest 87, 687–693 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowe SE, Sestini P & Perdue MH Allergic reactions of rat jejunal mucosa. Ion transport responses to luminal antigen and inflammatory mediators. Gastroenterology 99, 74–82 (1990). [DOI] [PubMed] [Google Scholar]
- 40.Thiagarajah JR, Donowitz M & Verkman AS Secretory diarrhoea: mechanisms and emerging therapies. Nature reviews. Gastroenterology & hepatology 12, 446–457, doi: 10.1038/nrgastro.2015.111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett KE & Keely SJ Chloride secretion by the intestinal epithelium: Molecular basis and Regulatory Aspects. Ann. Rev. Physiol. 62, 535–572 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Homaidan FR, Tripodi J, Zhao L & Burakoff R Regulation of ion transport by histamine in moue cecum. Eur. J. Pharmacol. 331, 199–204 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Kim YS et al. Histamine 1 receptor-Gbetagamma-cAMP/PKA-CFTR pathway mediates the histamine-induced resetting of the suprachiasmatic circadian clock. Molecular brain 9, 49, doi: 10.1186/s13041-016-0227-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kellum JM, Budhoo MR, Siriwardena AK, Smith EP & Jebraili SA Serotonin induces Cl- secretion in human jejunal mucosa in vitro via a nonneural pathway at a 5-HT4 receptor. The American journal of physiology 267, G357–363, doi: 10.1152/ajpgi.1994.267.3.G357 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Collins D, Hogan AM, Skelly MM, Baird AW & Winter DC Cyclic AMP-mediated chloride secretion is induced by prostaglandin F2alpha in human isolated colon. British journal of pharmacology 158, 1771–1776, doi: 10.1111/j.1476-5381.2009.00464.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura T et al. Mast cell-derived prostaglandin D2 attenuates anaphylactic reactions in mice. J Allergy Clin Immunol 140, 630–632.e639, doi: 10.1016/j.jaci.2017.02.030 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Wu D et al. Interleukin-13 (IL-13)/IL-13 Receptor α1 (IL-13Rα1) Signaling Regulates Intestinal Epithelial Cystic Fibrosis Transmembrane Conductance Regulator Channel-dependent Cl− Secretion*. J Biol Chem 286, 13357–13369, doi: 10.1074/jbc.M110.214965 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galietta LJ et al. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 168, 839–845 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Zund G, Madara JL, Dzus AL, Awtrey CS & Colgan SP Interleukin-4 and Interleukin-13 differentially regulate epithelial chloride secretion. J Biol Chem 271, 7460–7464 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Anthony RM, Rutitzky LI, Urban JF Jr., Stadecker MJ & Gause WC Protective immune mechanisms in helminth infection. Nat Rev Immunol 7, 975–987, doi: 10.1038/nri2199 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JB et al. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J Allergy Clin Immunol 137, 1216–1225.e1215, doi: 10.1016/j.jaci.2015.09.019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groschwitz KR & Hogan SP Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124, 3–20; quiz 21-22, doi:S0091-6749(09)00864-1 [pii] 10.1016/j.jaci.2009.05.038 [doi] (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner JR Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9, 799–809, doi:nri2653 [pii] 10.1038/nri2653 [doi] (2009). [DOI] [PubMed] [Google Scholar]
- 54.Turner MW et al. Intestinal hypersensitivity reactions in the rat. I. Uptake of intact protein, permeability to sugars and their correlation with mucosal mast-cell activation. Immunology 63, 119–124 (1988). [PMC free article] [PubMed] [Google Scholar]
- 55.King SJ, Miller HR, Newlands GF & Woodbury RG Depletion of mucosal mast cell protease by corticosteroids: effect on intestinal anaphylaxis in the rat. Proc Natl Acad Sci U S A 82, 1214–1218 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scudamore CL, Thornton EM, McMillan L, Newlands GF & Miller HR Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med 182, 1871–1881 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu LC & Perdue MH Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol Rev 179, 61–73 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Bischoff SC Mucosal allergy: role of mast cells and eosinophil granulocytes in the gut. Bailliere’s clinical gastroenterology 10, 443–459 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Gurish MF & Austen KF The diverse roles of mast cells. The Journal of experimental medicine 194, F1–5 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galli SJ, Maurer M & Lantz CS Mast cells as sentinels of innate immunity. Curr.Opin.Immunol 11, 53–59 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Pejler G, Abrink M, Ringvall M & Wernersson S Mast cell proteases. Advances in immunology 95, 167–255, doi:S0065-2776(07)95006-3 [pii] 10.1016/S0065-2776(07)95006-3 [doi] (2007). [DOI] [PubMed] [Google Scholar]
- 62.Bankova LG et al. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J Immunol 192, 2812–2820, doi: 10.4049/jimmunol.1301794 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence CE, Paterson YY, Wright SH, Knight PA & Miller HR Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology 127, 155–165, doi:S0016508504006092 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 64.Groschwitz KR et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A 106, 22381–22386, doi:0906372106 [pii] 10.1073/pnas.0906372106 [doi] (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Groschwitz KR, Wu D, Osterfeld H, Ahrens R & Hogan SP Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 304, G479–489, doi: 10.1152/ajpgi.00186.2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacob C et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem 280, 31936–31948 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Wilcz-Villega EM, McClean S & O’Sullivan MA Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. The American journal of gastroenterology 108, 1140–1151, doi: 10.1038/ajg.2013.92 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Scudamore CL et al. Basal secretion and anaphylactic release of rat mast cell protease-II (RMCP-II) from ex vivo perfused rat jejunum: translocation of RMCP-II into the gut lumen and its relation to mucosal histology. Gut 37, 235–241 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baniyash M & Eshhar Z Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol 14, 799–807 (1984). [DOI] [PubMed] [Google Scholar]
- 70.Capaldo CT et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Molecular biology of the cell 25, 2710–2719, doi: 10.1091/mbc.E14-02-0773 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


