Abstract
Background
Current guidelines recommend concomitant repair of certain non-severe cases of tricuspid regurgitation (TR) in patients undergoing cardiac surgery, but the prognostic relevance and postsurgical impact of the TR remain uncertain.
Objectives
The purpose of this study was to determine the prognostic impact of functional TR in patients undergoing diverse cardiac surgeries and to examine the effect-modifying role of patient characteristics in patients in whom TR confers a greater risk of adverse outcomes.
Methods
Patients undergoing coronary artery bypass, aortic, and mitral valve surgery were included. Patients with severe TR, organic tricuspid valve pathology, undergoing tricuspid valve surgery or without a recent preoperative echocardiogram were excluded. Clinical variables were extracted from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. An independent cohort was used for external validation.
Results
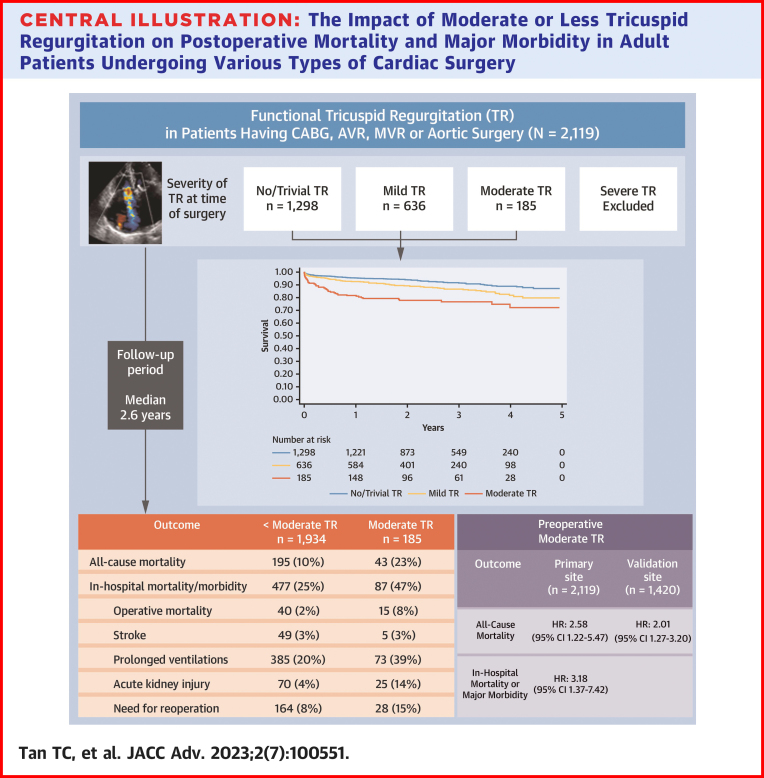
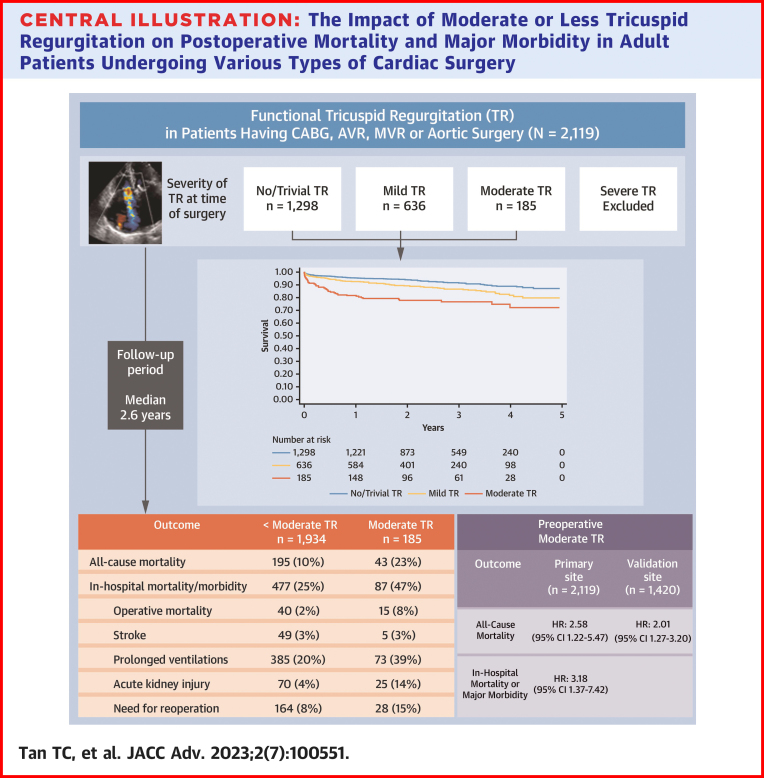
Of 2,119 patients (mean age 67.4 years; 29% females), TR severity was moderate in 185 (9%), mild in 636 (30%), trivial in 1,126 (53%), and absent in 172 (8%). There were 238 deaths during the median follow-up period of 2.6 years. After adjusting for relevant factors, moderate TR was found to be independently associated with mid-term mortality (HR: 2.58; 95% CI: 1.22-5.47) and with in-hospital mortality or major morbidity (OR: 3.18; 95% CI: 1.37-7.42). The association between TR and mortality was apparent when preoperative pulmonary artery systolic pressure was <40 mm Hg but not ≥40 mm Hg (P for interaction = 0.036).
Conclusions
In this diverse cohort of contemporary cardiac surgery patients, moderate functional TR was associated with increased mortality and major morbidity, particularly in the absence of pulmonary hypertension.
Key words: cardiac surgery, echocardiography, mortality, tricuspid regurgitation
Central Illustration
A sizeable proportion of patients requiring surgery for pathologies of the left heart manifest moderate-to-severe regurgitation of the tricuspid valve (TR) that is functional in nature.1,2 Functional TR occurs in the absence of organic tricuspid leaflet pathology and is caused by a variable combination of right atrial and tricuspid annular dilatation, right ventricular (RV) remodeling, and subvalvular tethering.3 These maladaptive changes disrupt the geometric alignment of the tricuspid apparatus and disallow it from achieving a sufficient amount of leaflet coaptation necessary to maintain valvular competency.4,5 Regardless of its contributing causes, the hemodynamic consequence of TR is characterized by volume overload of the RV and a vicious cycle of further dilatation and TR that can lead to chronic RV failure and death.6
The relevance of functional TR has been a topic of great debate, with initial reports suggesting that it was an innocuous byproduct that resolved after surgical correction of the primary left-sided heart pathology—most commonly mitral regurgitation or stenosis.7 Later reports employing more sensitive echocardiographic techniques to quantify TR argued that moderate-to-severe TR was, quite contrarily, associated with a heightened risk of mortality and morbidity if left untreated at the time of mitral valve surgery.6,8 Concerned about the risk of latent mortality and progressive TR requiring reoperation, North American and European guidelines have since recommended a more liberal approach to performing concomitant tricuspid valve surgery when TR is severe, or even when it is mild or moderate and deemed to have a high likelihood of progression (for example, when there is RV dysfunction or tricuspid annular dilatation).9, 10, 11
To date, the clinical practice of performing concomitant tricuspid valve repair for mild or moderate degrees of functional TR remains lower than would be expected based on these guidelines.11 The hesitancy stems from lingering doubts about the true prognostic impact of non-severe functional TR, with the evidence being particularly thin outside of the mitral valve literature. Indeed, recent data largely have supported concomitant tricuspid valve repair at the time of mitral surgery.12 However, there is lack of data to help clinicians differentiate cases of non-severe TR that will evolve favorably or unfavorably after left heart surgery.3 Thus, we sought to address these knowledge gaps by determining the prognostic impact of functional TR in a large contemporary cohort of patients undergoing diverse cardiac surgeries, examining the effect-modifying role of patient characteristics in identifying groups of patients in whom TR confers a greater risk of adverse outcomes. This is important particularly with advances in transcatheter therapies for the tricuspid valve.13,14
Methods
Study design
A cohort of consecutive patients undergoing coronary artery bypass grafting (CABG), surgical repair or replacement of the aorta, aortic valve replacement (AVR), or mitral valve replacement (MVR) at the Massachusetts General Hospital (MGH) in the last decade was evaluated. Preoperative echocardiograms were analyzed to determine whether functional TR was associated with postoperative mortality or major morbidity during the index hospitalization and during the subsequent follow-up period. The study protocol was approved by Institutional Review Board, which waved the requirement for patient-level informed consent. The manuscript was prepared in accordance with the Strengthening of Reporting of Observational Studies in Epidemiology guidelines.15
Selection criteria
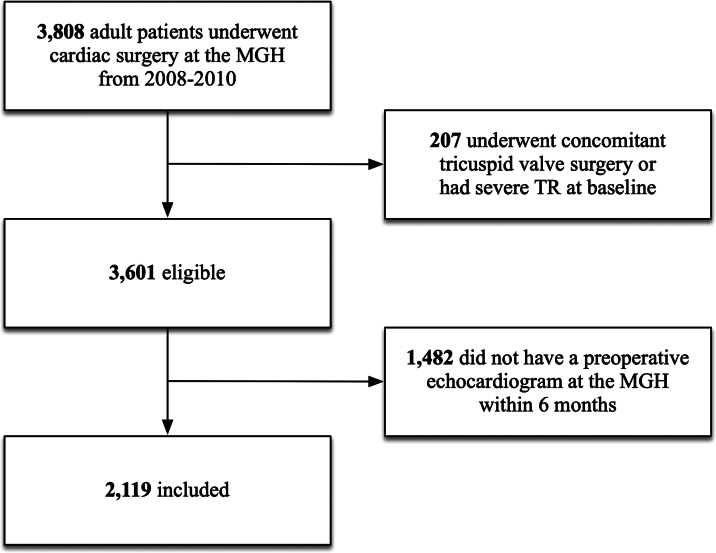
Patients who underwent CABG, MVR, AVR, or aortic surgery, either in isolation or in combination, were eligible to be included. Patients who underwent concomitant or prior tricuspid valve repair or replacement surgery were excluded, as were those with organic tricuspid valve pathology or severe TR at baseline. The rationale for these exclusions was to focus solely on the controverted implications of unrepaired non-severe functional TR (with the prognostic and therapeutic implications of severe TR being generally accepted). Since echocardiographic images were reanalyzed by core readers for the purposes of this study, patients who did not have an available echocardiogram in digital format from the MGH Cardiac Ultrasound Lab within 6 months before the time of cardiac surgery were excluded.
Data collection
Demographics, comorbidities, surgical approach, and complications were extracted from the Society for Thoracic Surgeons (STS) Adult Cardiac Surgery Database and the Research Patient Data Registry (Partners Healthcare, Boston, Massachusetts, USA). Patients were followed from the time of hospital admission to discharge, and then followed forward for vital status. Vital status data were extracted from the National Social Security Death Index. The primary outcome was all-cause death occurring from the time of cardiac surgery to the end of follow-up. The secondary outcome was a composite of in-hospital mortality or major morbidity defined according to the STS as all-cause death, stroke, acute kidney failure, prolonged ventilation, or reoperation.
Echocardiographic assessment
Echocardiograms were acquired with Philips IE33, Sonos 7500 or GE Vivid 7 machines and analyzed on Xcelera (Philips Healthcare) workstations. Image analysis was performed by level III-trained echocardiography readers according to the guidelines of the American Society of Echocardiography.16,17 Valvular regurgitation, notably TR, was quantified as absent, trace, mild, moderate, or severe based on a multi-parametric approach integrating color Doppler jet appearance, hepatic vein Doppler pattern, and vena contracta width or proximal isovelocity shell area when technically feasible. Pulmonary artery systolic pressure (PASP) was calculated as: 4 × TR velocity2 plus right atrial pressure depending on the inferior vena cava size and collapse.
External validation
A separate cohort of patients undergoing CABG, MVR, surgical or transcatheter AVR, and aortic surgery at McGill University hospitals (Jewish General Hospital, Royal Victoria Hospital [JGH]) between 2008 and 2020 was evaluated to confirm the effect size of moderate TR. Again, patients who had severe TR or underwent tricuspid valve repair or replacement were excluded. Demographics, comorbidities, surgical approach, and complications were extracted from the McGill Frailty Registry. Echocardiograms were acquired with the same type of equipment from Philips and GE, and TR was quantified with the same standardized criteria; however, PASP was calculated as 4 × TR velocity2 + 3, 8, or 15 mm Hg for right atrial pressure depending on the inferior vena cava size and collapse. Vital status data were extracted from the electronic health record, telephone follow-up with patients, or next of kin.
Statistical methods
Baseline variables were compared between patients with moderate and less than moderate TR using the chi-squared test for categorical variables and Wilcoxon rank-sum test for continuous variables. Multivariable logistic and Cox regression models were used to determine the effect of TR on the primary and secondary outcome measures. The proportional hazards assumption was explored by visual assessment of KM curves, log-minus-log plots, and testing of scaled Schoenfeld residuals.
Models were adjusted for age, sex, myocardial infarction, atrial fibrillation, permanent pacemaker, stroke, chronic lung disease, serum creatinine, type of surgical procedure, surgical urgency, prior cardiac surgery, and echocardiographic covariates: TR, mitral regurgitation severity, left ventricular ejection fraction (LVEF), RV dysfunction, and PASP. Variables known to be associated with TR progression and adverse outcomes were examined with the use of stratified models and interaction terms to determine whether the prognostic impact of TR varied according to the presence or absence of these factors. Analyses were performed with the STATA 14 software package.
Results
The cohort consisted of 2,119 patients with a mean age of 67.4 ± 12.9 years and 29% females. TR severity was moderate in 185 (9%), mild or mild-to-moderate in 636 (30%), trivial in 1,126 (53%), and absent in 172 (8%). The prevalence of unrepaired moderate TR was highest in patients undergoing MVR (19%), followed by AVR and aortic surgery (12%), and CABG (4%). Baseline characteristics for patients with moderate TR vs <moderate TR are shown in Table 1. Patients with moderate TR were more likely to be older, female, have lower LVEF, higher PASP, RV dysfunction, atrial fibrillation, prior pacemaker, chronic lung disease, and undergo MVR or redo surgery. The STS-predicted risk of mortality was 8.5% ± 5.9% in those with moderate TR as compared to 3.7% ± 5.1% in those with <moderate TR. The main reason for exclusion was not having a recent preoperative echocardiogram at our ultrasound lab (Figure 1); these patients tended to be referred from outside hospitals and have younger age, lower comorbidity burden, and lower risk of mortality (Supplemental Table 1).
Table 1.
Baseline Characteristics by TR Grade
| <Moderate TR | Moderate TR | |
|---|---|---|
| (n = 1,934) | (n = 185) | |
| Age, y | 66.9 ± 12.8 | 72.0 ± 13.0 |
| Female | 535 (28%) | 90 (49%) |
| Myocardial infarction | 849 (44%) | 81 (44%) |
| Atrial fibrillation | 315 (16%) | 75 (41%) |
| Permanent pacemaker | 82 (4%) | 32 (17%) |
| Chronic lung disease | 285 (15%) | 43 (23%) |
| Diabetes | 598 (31%) | 56 (30%) |
| Stroke | 171 (9%) | 17 (9%) |
| Serum creatinine, mmol/L | 112.6 ± 68.8 | 123.0 ± 63.9 |
| TR grade,/4 | 1.2 ± 0.6 | 3.0 ± 0.0 |
| MR grade,/4 | 1.7 ± 0.9 | 2.6 ± 0.9 |
| LVEF, % | 57.8 ± 13.8 | 51.4 ± 17.8 |
| RV dysfunction | 134 (7%) | 47 (25%) |
| PASP | 39.1 ± 11.5 | 55.7 ± 15.5 |
| ≥40 mm Hg | 580 (30%) | 157 (85%) |
| ≥50 mm Hg | 225 (12%) | 115 (62%) |
| Urgent surgery | 1,210 (63%) | 125 (68%) |
| Redo surgery | 188 (10%) | 52 (28%) |
| CABG | 1,342 (69%) | 82 (44%) |
| AVR | 704 (36%) | 85 (46%) |
| MVR | 180 (9%) | 42 (23%) |
| Aortic surgery | 309 (16%) | 47 (25%) |
| STS-predicted risk of mortality, % | 3.7 ± 5.1 | 8.5 ± 5.9 |
Values are mean ± SD or n (%).
AVR = aortic valve replacement or repair; CABG = coronary artery bypass graft; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; MVR = mitral valve replacement or repair; PASP = pulmonary artery systolic pressure; RV = right ventricle; STS = Society of Thoracic Surgeons; TR = tricuspid regurgitation.
Figure 1.
Flow Diagram
MGH = Massachusetts General Hospital; TR = tricuspid regurgitation.
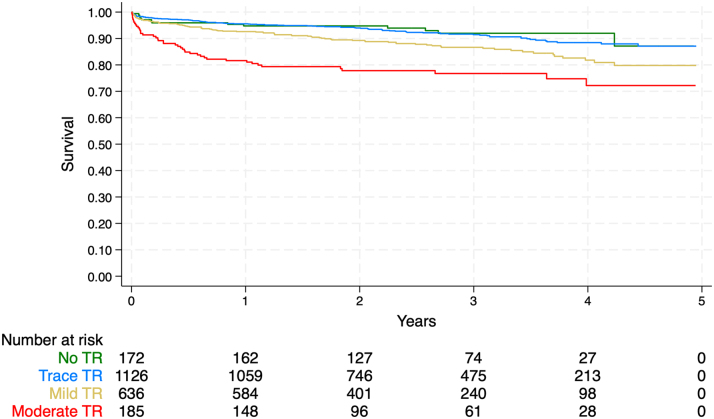
The cohort was followed for a mean of 2.6 ± 1.3 years, during which time 238 all-cause deaths were observed, representing 23% of patients with moderate TR and 10% of patients with <moderate TR (unadjusted HR: 2.57; 95% CI: 1.84-3.59) (Table 2, Figure 2). During the index hospitalization, 564 composite deaths or major morbidities were observed, representing 47% of patients with moderate TR and 25% of patients with <moderate TR (unadjusted OR: 2.71; 95% CI: 2.00-3.68). In multivariable models adjusted for age, sex, comorbid conditions, surgical, and echocardiographic covariates, moderate TR remained independently associated with mid-term mortality (adjusted HR: 2.58; 95% CI: 1.22-5.47) (Table 3) and in-hospital mortality or major morbidity (adjusted OR: 3.18; 95% CI: 1.37-7.42) (Table 4).
Table 2.
Postoperative Outcomes by TR Grade
| <Moderate TR(n = 1,934) | Moderate TR(n = 185) | |
|---|---|---|
| All-cause mortality | 195 (10%) | 43 (23%) |
| In-hospital mortality/morbidity | 477 (25%) | 87 (47%) |
| Operative mortality | 40 (2%) | 15 (8%) |
| Stroke | 49 (3%) | 5 (3%) |
| Prolonged ventilations | 385 (20%) | 73 (39%) |
| Acute kidney injury | 70 (4%) | 25 (14%) |
| Need for reoperation | 164 (8%) | 28 (15%) |
Values are n (%).
TR = tricuspid regurgitation.
Figure 2.
Kaplan-Meier Curves for All-Cause Mortality by TR Grade
TR = tricuspid regurgitation.
Table 3.
Cox Regression Model for All-Cause Mortality Over 2.6 Years
| Age, per y | 1.05 (1.04-1.07) |
| Female | 1.09 (0.82-1.45) |
| Myocardial infarction | 1.26 (0.94-1.69) |
| Atrial fibrillation | 1.52 (1.12-2.05) |
| Permanent pacemaker | 1.14 (0.72-1.83) |
| Chronic lung disease | 1.99 (1.50-2.66) |
| Diabetes | 1.64 (1.25-2.15) |
| Stroke | 0.81 (0.52-1.26) |
| Serum creatinine, per mg/dL | 1.37 (1.25-1.49) |
| Urgent surgery | 1.22 (0.89-1.67) |
| Redo surgery | 1.02 (0.68-1.54) |
| CABG | 1.18 (0.83-1.68) |
| AVR | 1.16 (0.86-1.57) |
| MVR | 1.30 (0.82-2.05) |
| Aortic surgery | 1.74 (1.20-2.51) |
| LVEF, per % | 0.99 (0.98-0.99) |
| RV dysfunction | 1.14 (0.75-1.72) |
| Moderate MR | 1.18 (0.84-1.66) |
| Moderate TR | 2.58 (1.22-5.47) |
| PASP ≥40 mm Hg | 1.43 (1.06-1.94) |
| Interaction term for TR and PASP | P = 0.036 |
Values are adjusted HR (95% CI).
AVR = aortic valve replacement or repair; CABG = coronary artery bypass graft; CI = confidence interval; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; MVR = mitral valve replacement or repair; PASP = pulmonary artery systolic pressure; RV = right ventricle; TR = tricuspid regurgitation.
Table 4.
Logistic Regression Model for In-Hospital Mortality or Major Morbidity
| Age, per y | 1.02 (1.01-1.03) |
| Female | 1.19 (0.94-1.51) |
| Myocardial infarction | 1.07 (0.85-1.36) |
| Atrial fibrillation | 1.37 (1.05-1.79) |
| Permanent pacemaker | 1.28 (0.82-1.99) |
| Chronic lung disease | 1.41 (1.08-1.86) |
| Diabetes | 1.08 (0.86-1.36) |
| Stroke | 1.15 (0.81-1.64) |
| Serum creatinine, per mg/dL | 1.12 (0.99-1.27) |
| Urgent surgery | 2.19 (1.70-2.82) |
| Redo surgery | 1.47 (1.05-2.07) |
| CABG | 2.26 (1.65-3.07) |
| AVR | 1.99 (1.54-2.57) |
| MVR | 1.99 (1.31-3.03) |
| Aortic surgery | 2.65 (1.93-3.63) |
| LVEF, per % | 0.99 (0.99-1.00) |
| RV dysfunction | 1.79 (1.23-2.59) |
| Moderate MR | 1.08 (0.79-1.47) |
| Moderate TR | 3.18 (1.37-7.42) |
| PASP ≥40 mm Hg | 1.19 (0.93-1.53) |
| Interaction term for TR and PASP | P = 0.059 |
Values are adjusted OR (95% CI).
AVR = aortic valve replacement or repair; CABG = coronary artery bypass graft; CI = confidence interval; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; MVR = mitral valve replacement or repair; PASP = pulmonary artery systolic pressure; RV = right ventricle; TR = tricuspid regurgitation.
Estimated PASP was 55.7 ± 15.5 mm Hg in patients with moderate TR as compared to 39.1 ± 11.5 mm Hg in patients with <moderate TR. In stratified multivariable models, the predictive effect of moderate TR on mid-term mortality was apparent when PASP was <40 mm Hg (adjusted HR: 2.30; 95% CI: 1.04-5.10) but not ≥40 mm Hg (adjusted HR: 1.09; 95% CI: 0.71-1.67). The interaction term combining TR severity and PASP was statistically significant (P = 0.0036). Sensitivity analyses using a different PASP cutoff of 50 mm Hg yielded similar results. Of note, the prevalence of baseline RV dysfunction was similar in patients with PASP <40 vs ≥40 mm Hg (21% vs 26%; P = 0.60). The predictive effect of moderate TR was more apparent when RV dysfunction was present (adjusted HR: 2.11 vs 1.20) although this interaction term was not statistically significant (P = 0.65). There was no evidence of interaction for the other candidate effect modifiers, namely: age, atrial fibrillation, LVEF, and type of surgical procedure.
External validation cohort
The McGill cohort consisted of 1,420 patients with a mean age of 73.1 ± 9.7 years and 31% females (Supplemental Tables 2 and 3). The distribution of procedures performed was similar in the McGill vs MGH cohorts other than a lower proportion of surgical AVR (27% vs 37%) and a higher proportion of transcatheter AVR (22% vs 0%). TR severity was moderate in 62 (4%), mild or mild-to-moderate in 527 (37%), trivial or absent in 831 (59%). The McGill cohort was followed for a mean of 3.5 ± 2.3 years, during which time 280 all-cause deaths were observed, representing 42% of patients with moderate TR and 19% of patients with <moderate TR (unadjusted HR: 3.50; 95% CI: 2.33-5.25). In a multivariable model adjusted for age, sex, comorbid conditions, surgical, and echocardiographic covariates, moderate TR remained independently associated with mid-term mortality (adjusted HR: 2.01; 95% CI: 1.27-3.20) (Supplemental Table 4) with no evidence of interaction.
Discussion
This study has highlighted the negative prognostic implications of functional TR in a large real-world sample of patients undergoing cardiac surgery. Moderate TR was associated with a 2- to 3-fold increase in mortality and major morbidity during the in-hospital and subsequent mid-term follow-up period. One of the novel findings was the interaction between TR and PASP, whereby patients with moderate TR and normal PASP were found to have a markedly greater risk of mortality. Patients with moderate TR and RV dysfunction were also found to confer greater risk, although this did not achieve statistical significance in formal interaction testing. Thus, the prognostic implications of functional TR varied depending on the presence or absence of key effect modifiers identified in this study (Central Illustration).
Central Illustration.
The Impact of Moderate or Less Tricuspid Regurgitation on Postoperative Mortality and Major Morbidity in Adult Patients Undergoing Various Types of Cardiac Surgery
Previous studies have reported an association between TR and adverse outcomes after cardiac surgery.1,3,18,19 These studies were focused on narrow patient groups undergoing either mitral or aortic valve interventions. A seminal publication by Sagie20 studied 318 patients undergoing percutaneous balloon mitral valvuloplasty for mitral stenosis and found TR to be among the most important predictors of procedural outcomes and 4-year mortality. Di Mauro21 and Chan22 studied 165 and 624 patients undergoing MVR and found moderate TR to be an independent predictor of 5-year mortality (HR: 1.5-3.1). Mascherbauer23 and Lindman24 studied 465 patients undergoing surgical AVR and 542 patients undergoing transcatheter AVR, respectively, and again found moderate TR be an independent predictor of mid-term mortality (HR: 2.5-3.2). The GRASP Registry (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation Registry)25 presented data on 146 patients undergoing the transcatheter MitraClip procedure and found that ≥moderate TR was one of 2 variables independently predictive of symptomatic improvement and 1-year mortality or readmission (HR: 2.7). The effect observed in our study is entirely consistent with the aforementioned studies and has extended the results to a larger sample size and broader case mix of mitral, aortic, and coronary surgeries.
Our study has added new insights into the important role of preoperative PASP on modulating the downstream impact of functional TR. Moderate TR conferred a greater risk of latent mortality in patients with normal PASP and a nonsignificant risk in those with elevated PASP as demonstrated by the statistically significant interaction and by the PASP-stratified hazard ratios. Lindman noted a similar signal in his analysis of patients undergoing transcatheter AVR, with ≥moderate TR conferring a HR of 3.5 when mean pulmonary artery pressure was <35 mm Hg and a nonsignificant HR of 2.0 when ≥35 mm Hg.24 This finding is in counter-current to the Class IIb guideline recommendation to consider performing concomitant tricuspid valve repair in patients undergoing cardiac surgery who have mild-to-moderate TR when PASP is elevated.10
The rationale for this recommendation remains elusive and is not clearly supported in the guidelines but may stem from the belief that TR is more likely to progress in the presence of pulmonary hypertension. While the 2020 American College of Cardiology/American Heart Association guidelines emphasize elevated PASP as a potential indication for concomitant repair of moderate TR, our study did not identify elevated PASP as an effect modifier for worse outcomes in either the primary MGH cohort or the secondary JGH cohort; calling into question the validity of this indication. To the contrary, it was the patients with moderate TR and normal PASP that appeared to have worse outcomes, although this was not replicated in the secondary JGH cohort and thus remains hypothesis generating at this time.
Numerous studies have examined the risk factors for functional TR progression after MVR and failed to substantiate this belief that higher baseline PASP is associated with TR progression over time. The risk factors for TR progression most consistently identified in these studies were advanced age, atrial fibrillation, RV dysfunction, dilated tricuspid annulus, rheumatic or ischemic mitral valve disease, and reduced LVEF.26 In patients undergoing surgery for functional TR, an acceptable early and late mortality was reported.19 However, TR remains prevalent after surgery.14
Why could moderate TR imply a greater risk in the face of normal PASP? One postulated reason is that the failing RV cannot generate a high PASP, such that a low or normal PASP may be a surrogate indicator for advanced RV systolic dysfunction. In our cohort, the echocardiographic prevalence of RV systolic dysfunction was 21% with a mean fractional area change of 0.45 in patients with moderate TR and PASP <40 mm Hg as compared to 26% and 0.41 in patients with moderate TR and PASP ≥40 mm Hg. Therefore, since RV systolic function was similar in both groups (and it was LV diastolic function and left atrial pressure that were more abnormal in the PASP ≥40 mm Hg group), this is unlikely to be the primary reason. Another postulated reason is that patients with high baseline PASP are likely to benefit from some degree of postoperative reduction in PASP and a consequent improvement in TR severity after correction of their left heart pathology.
The magnitude of the postoperative reduction in PASP has been shown to be an important predictor of functional TR regression after MVR for patients with mitral valve disease.27 Patients with normal baseline PASP are less likely to expect this type of improvement in TR after surgery and are more likely to have other nefarious mechanisms that are responsible for functional TR and its progression.28 These mechanisms include RV remodeling from interventricular interactions, RV infarction, or right atrial and tricuspid annular dilatation from chronic atrial fibrillation.3,29,30 Dilatation of the right heart chambers and TR perpetuate a vicious cycle of RV volume overload and progressive TR. Accordingly in our cohort, moderate TR was associated with a greater incremental risk of mortality in the setting of RV dysfunction and in the setting of aortic surgery, which corresponded to the patient subgroups with the lowest preoperative prevalence of pulmonary hypertension.
Study limitations
Echocardiograms were not systematically performed after cardiac surgery to document the postoperative changes in TR severity and PASP; while it would have been interesting to obtain mechanistic insights, it would be better addressed by a prospective study design with serial echocardiograms performed at predefined time intervals. The second limitation is that tricuspid annular dimensions were not routinely measured; an indexed dimension of ≥21 mm/m2 has been shown to be one of the most important predictors of TR progression after cardiac surgery and represents a Class IIa guideline recommendation to perform concomitant tricuspid valve repair in patients with mild or moderate TR.10 Other than tricuspid annular diameter, most of the pertinent clinical and echocardiographic effect modifiers were collected and analyzed. The third limitation is that long-term follow-up >5 years was not available and may have been informative to appreciate the negative effects of progressive TR over time. Most patients in our study were followed for 2 to 4 years, which compares favorably with previous studies in the field. Moreover, it is reassuring to note that progression of untreated non-severe TR is far less common (<5%-10% of cases) than stability or regression after aortic or mitral valve surgery.31,32
Conclusions
In our representative sample of adult patients undergoing various types of cardiac surgery, the echocardiographic finding of moderate TR heralded a clinically important increase in risk of postoperative mortality and major morbidity. The increase in risk was magnified when moderate TR was accompanied by a normal PASP and to a lesser extent by a dysfunctional RV. Whereas previous studies focused on the implications of TR in mitral or aortic valve disease, our results indicate that the implications may be as significant if not more in other types of procedures such as aortic surgery. Clinical trials are needed to determine whether concomitant tricuspid valve repair should be advocated in cases when TR is moderate and PASP is normal.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Functional tricuspid regurgitation is associated with a heightened risk of mortality and major morbidity after cardiac surgery.
COMPETENCY IN PATIENT CARE: Concomitant repair of functional tricuspid regurgitation should be considered when it is severe or progressive, the latter being more likely when pulmonary artery systolic pressure is normal or right ventricular function is impaired.
TRANSLATIONAL OUTLOOK: The mechanistic reasons for tricuspid regurgitation progression and adverse clinical outcomes require further investigation. Serial examinations of tricuspid valve mobility and competency should be related to atrioventricular remodeling and pulmonary arterial hemodynamics.
Funding support and author disclosures
Dr Afilalo was supported by the FRSQ (Fonds de Recherché Santé du Québec) Research Fellowship Award. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to thank Marcia Leavitt and David Crowell for their invaluable help in obtaining clinical and echocardiographic data for this study. They also thank all of the cardiac sonographers at the Massachusetts General Hospital for their excellence in acquiring the echocardiographic images that made this study possible.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Supplementary data
References
- 1.Kelly B.J., Ho Luxford J.M., Butler C.G., et al. Severity of tricuspid regurgitation is associated with long-term mortality. J Thorac Cardiovasc Surg. 2018;155:1032–1038.e2. doi: 10.1016/j.jtcvs.2017.09.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benfari G., Antoine C., Miller W.L., et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. 2019;140:196–206. doi: 10.1161/CIRCULATIONAHA.118.038946. [DOI] [PubMed] [Google Scholar]
- 3.Essayagh B., Antoine C., Benfari G., et al. Functional tricuspid regurgitation of degenerative mitral valve disease: a crucial determinant of survival. Eur Heart J. 2020;41:1918–1929. doi: 10.1093/eurheartj/ehaa192. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfus G., Martin R., Chan K., Dulguerov F., Alexandrescu C. Functional tricuspid regurgitation. J Am Coll Cardiol. 2015;65:2331. doi: 10.1016/j.jacc.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Arsalan M., Walther T., Smith R.L., Grayburn P.A. Tricuspid regurgitation diagnosis and treatment. Eur Heart J. 2017;38:634–638. doi: 10.1093/eurheartj/ehv487. [DOI] [PubMed] [Google Scholar]
- 6.Nath J., Foster E., Heidenreich P.A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald N.S., Ross J., Jr., Morrow A.G. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation. 1967;35:I63–I69. doi: 10.1161/01.cir.35.4s1.i-63. [DOI] [PubMed] [Google Scholar]
- 8.Bannehr M., Edlinger C.R., Kahn U., et al. Natural course of tricuspid regurgitation and prognostic implications. Open Heart. 2021;8 doi: 10.1136/openhrt-2020-001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 10.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Tourmousoglou C. Is the diameter of tricuspid annulus or functional tricuspid regurgitation the key parameter for performing ‘prophylactic annuloplasty’? Eur J Cardio Thorac Surg. 2020;57(1):203. doi: 10.1093/ejcts/ezz066. [DOI] [PubMed] [Google Scholar]
- 12.Patel K.M., Kumar N.S., Neuburger P.J., Desai R.G., Krishnan S. Functional tricuspid regurgitation in patients with chronic mitral regurgitation: an evidence-based narrative review. J Cardiothorac Vasc Anesth. 2022;36(6):1730–1740. doi: 10.1053/j.jvca.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Orban M., Rommel K.-P., Ho E.C., et al. Transcatheter edge-to-edge tricuspid repair for severe tricuspid regurgitation reduces hospitalizations for heart failure. Heart Fail. 2020;8:265–276. doi: 10.1016/j.jchf.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Taramasso M., Gavazzoni M., Pozzoli A., et al. Tricuspid regurgitation: predicting the need for intervention, procedural success, and recurrence of disease. J Am Coll Cardiol Img. 2019;12:605–621. doi: 10.1016/j.jcmg.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Malta M., Cardoso L.O., Bastos F.I., Magnanini M.M.F., Silva C.M.F.P.D. Strobe initiative: guidelines on reporting observational studies. Rev Saude Publica. 2010;44:559–565. doi: 10.1590/s0034-89102010000300021. [DOI] [PubMed] [Google Scholar]
- 16.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Koren O., Darawsha H., Rozner E., Benhamou D., Turgeman Y. Tricuspid regurgitation in ischemic mitral regurgitation patients: prevalence, predictors for outcome and long-term follow-up. BMC Cardiovasc Disord. 2021;21:199. doi: 10.1186/s12872-021-01982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veen K.M., Etnel J.R., Quanjel T.J., et al. Outcomes after surgery for functional tricuspid regurgitation: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2020;6:10–18. doi: 10.1093/ehjqcco/qcz032. [DOI] [PubMed] [Google Scholar]
- 20.Sagie A., Schwammenthal E., Newell J.B., et al. Significant tricuspid regurgitation is a marker for adverse outcome in patients undergoing percutaneous ballon mitral valvuloplasty. J Am Coll Cardiol. 1994;24:696–702. doi: 10.1016/0735-1097(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 21.Di Mauro M., Di Giammarco G., Vitolla G., et al. Impact of no-to-moderate mitral regurgitation on late results after isolated coronary artery bypass grafting in patients with ischemic cardiomyopathy. Ann Thorac Surg. 2006;81:2128–2134. doi: 10.1016/j.athoracsur.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 22.Chan V., Burwash I.G., Lam B.-K., et al. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg. 2009;88:1209–1215. doi: 10.1016/j.athoracsur.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Mascherbauer J., Kammerlander A.A., Marzluf B.A., Graf A., Kocher A., Bonderman D. Prognostic impact of tricuspid regurgitation in patients undergoing aortic valve surgery for aortic stenosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindman B.R., Maniar H.S., Jaber W.A., et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the placement of aortic transcatheter valves ii inoperable cohort. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scandura S., Capranzano P., Caggegi A., et al. Percutaneous mitral valve repair with the mitraclip system in the elderly: one-year outcomes from the grasp registry. Int J Cardiol. 2016;224:440–446. doi: 10.1016/j.ijcard.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 26.Zhu T.-Y., Min X.-P., Zhang H.-B., Meng X. Preoperative risk factors for residual tricuspid regurgitation after isolated left-sided valve surgery: a systematic review and meta-analysis. Cardiology. 2014;129:242–249. doi: 10.1159/000367589. [DOI] [PubMed] [Google Scholar]
- 27.Shiran A., Najjar R., Adawi S., Aronson D. Risk factors for progression of functional tricuspid regurgitation. Am J Cardiol. 2014;113:995–1000. doi: 10.1016/j.amjcard.2013.11.055. [DOI] [PubMed] [Google Scholar]
- 28.Mutlak D., Aronson D., Lessick J., Reisner S.A., Dabbah S., Agmon Y. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest. 2009;135:115–121. doi: 10.1378/chest.08-0277. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz-Leon X.A., Posada-Martinez E.L., Trejo-Paredes M.C., et al. Understanding tricuspid valve remodelling in atrial fibrillation using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:747–755. doi: 10.1093/ehjci/jeaa058. [DOI] [PubMed] [Google Scholar]
- 30.Utsunomiya H., Itabashi Y., Mihara H., et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.004897. [DOI] [PubMed] [Google Scholar]
- 31.Jeong D.S., Sung K., Kim W.S., et al. Fate of functional tricuspid regurgitation in aortic stenosis after aortic valve replacement. J Thorac Cardiovasc Surg. 2014;148:1328–1333.e1. doi: 10.1016/j.jtcvs.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y., Li S., Zhuang X., Gao F., Shi L., Meng X. Comparison of mitral valve repair versus replacement for the progression of functional tricuspid regurgitation. Ann Thorac Cardiovasc Surg. 2020;26:72–78. doi: 10.5761/atcs.oa.19-00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.