Abstract
Background
Statins are highly effective for primary prevention of atherosclerotic cardiovascular disease (ASCVD) and mortality. Data on the benefit of statins in adults with heart failure with preserved ejection fraction (HFpEF) and without ASCVD are limited.
Objectives
The purpose of this study was to determine whether statins are associated with a lower risk of mortality and major adverse cardiovascular events (MACE) in HFpEF.
Methods
Veterans Health Administration data from 2002 to 2016, linked to Medicare and Medicaid claims and pharmaceutical data, were collected. Patients had a new HFpEF diagnosis and no known ASCVD or prior statin use at baseline. Cox proportional hazards models were fit to evaluate the association of new statin use with outcomes (all-cause mortality and MACE). Propensity score overlap weighting (PSW) was used to balance baseline characteristics.
Results
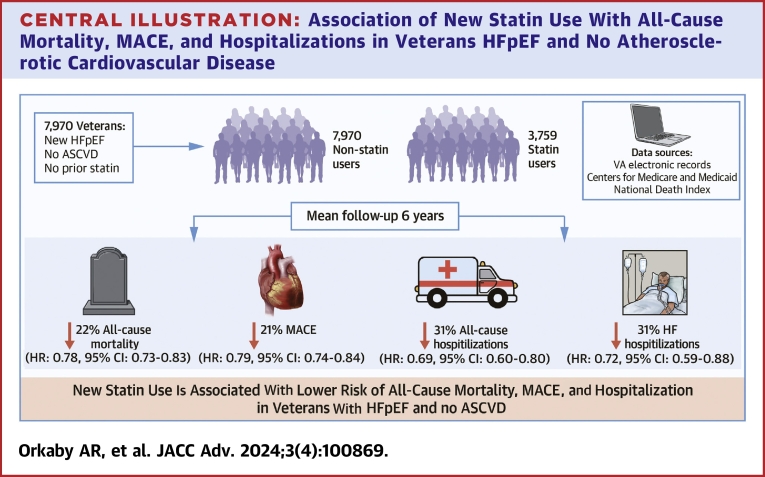
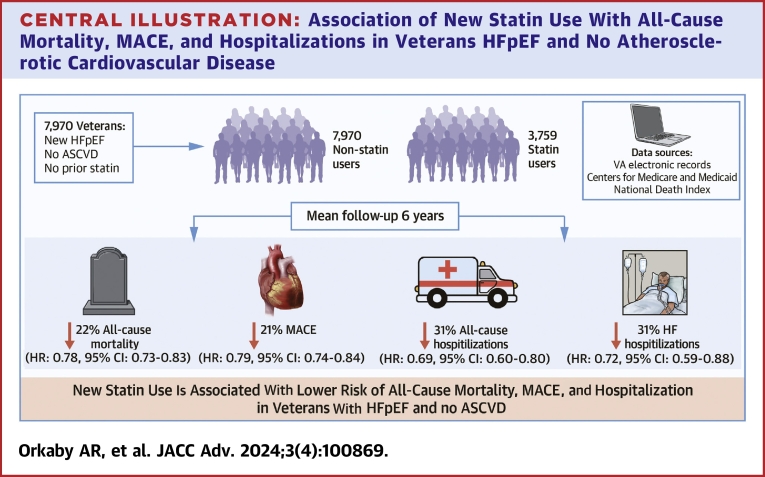
Among 7,970 Veterans, 47% initiated a statin over a mean 6.0-year follow-up. At HFpEF diagnosis, mean age was 69 ± 12 years, 96% were male, 67% were White, 14% were Black, and mean EF was 60% ± 6%. Before PSW, statin users were younger with more prevalent metabolic syndrome, arthritis, and other chronic conditions. All characteristics were balanced after PSW. There were 5,314 deaths and 4,859 MACE events. After PSW, the hazard for all-cause mortality for statin users vs nonusers was 22% lower (HR: 0.78; 95% CI: 0.73-0.83). The HR for MACE was 0.79 (95% CI: 0.74-0.84), 0.69 (95% CI: 0.60-0.80) for all-cause hospitalization, and 0.72 (95% CI: 0.59-0.88) for HF hospitalization.
Conclusions
New statin use was associated with reduced all-cause mortality, MACE, and hospitalization in Veterans with HFpEF without prevalent ASCVD.
Key words: heart failure with preserved ejection fraction, primary prevention, statins
Central Illustration
Statins are highly effective cholesterol-lowering medications used for the prevention of atherosclerotic cardiovascular disease (ASCVD)1 and are recommended for both primary and secondary prevention of ASCVD.2,3 However, the benefits of statins in individuals with heart failure (HF) are uncertain and remain an area of ongoing debate.4 Patients with HF were excluded from many of the early landmark studies examining the efficacy of statins;5 and the 2 major randomized controlled trials (RCTs) of statins specifically in participants with pre-existing HF did not improve outcomes. Indeed, in the CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) study, rosuvastatin failed to improve the composite endpoint of cardiovascular death, nonfatal myocardial infarction (MI), or fatal stroke compared to placebo over a median of 32.8 month;6 and in the GISSI-HF (Rosuvastatin in patients with chronic heart failure) trial,7 rosuvastatin failed to improve time to death, or time to death or cardiovascular hospitalization (coprimary endpoints) compared to placebo. Based on these studies, professional cardiology societies currently do not recommend statins for HF outside of other indications such as coronary artery disease.8,9
Notably, the CORONA and GISSI-HF trials primarily included HF with reduced ejection fraction (HFrEF) participants. Consequently, the role of statins in HF with preserved ejection fraction (HFpEF) is unclear. Given the role of inflammation in the pathophysiology of HFpEF,10 statins have been suggested as a potentially important pharmacologic therapy for a condition that until recently has had few therapeutic options.10 Although several observational studies have suggested statins may be beneficial in HFpEF, inferences from these studies have been limited by sample size, narrow geographic representation, and limited attempts to address confounding by indication.11, 12, 13 Using the national Veterans Health Administration (VA) data, we sought to examine a large cohort of patients with HFpEF to generate high-level observational data on the association of statins with mortality and ASCVD events in HFpEF.
Methods
Study population
We utilized the entire VA clinical database, which includes veterans seen across the 50 U.S. states and Puerto Rico, to curate an HFpEF cohort using a validated algorithm applied to electronic medical record data from 2002 to 2012, as previously described.14,15 Briefly, using claims data, the clinical record, and natural language processing tools, we developed an algorithm to identify cases of HFpEF. This included: 1) International Classification of Diseases-9th Revision codes diagnosis of HF (any 428.xx); 2) either B-type natriuretic peptide or amino terminal pro-B-type natriuretic peptide values recorded or diuretic usage within 1 month of HF diagnosis; 3) an echocardiogram in VA; and 4) all documented ejection fraction values ≥50%, consistent with clinical practice guidelines.4 Veterans with International Classification of Diseases-9th Revision codes for constrictive pericarditis, hypertrophic cardiomyopathy, and any prior history of HF were excluded. In total, 80,248 Veterans were identified as having HFpEF.
To conduct this study on primary prevention of ASCVD, we excluded Veterans with claims indicating prior coronary artery bypass graft or percutaneous coronary intervention, or who had claims indicating prior MI, stroke/transient ischemic attack, or peripheral vascular disease. VA data were merged with Medicare/Medicaid data to ensure complete capture of events, comorbidities, and medication use.
To remove the possibility of historic statin use influencing study results, we took a new-user approach16,17 and excluded Veterans with any statin use prior to HFpEF diagnosis. Therefore, at baseline, the entire study cohort began as non-statin users, free of ASCVD. Veterans entered the cohort with a new diagnosis of HFpEF from 2002 to 2012 and were followed through April 8, 2016.
Exposure
The primary exposure was new statin use. Medication data were extracted from VA pharmacy records and augmented with Medicare claims data. All statins approved for use in the United States were included: atorvastatin, cerivastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin.
Statin initiation was calculated from the first outpatient statin prescription fill after HFpEF diagnosis date; inpatient prescriptions were excluded. The entire cohort was considered statin nonusers at baseline, and at baseline began contributing person-time as statin nonusers. Once a statin was initiated, a Veteran switched and began contributing time as a statin user.
Outcome
We studied 2 coprimary outcomes: 1) all-cause mortality; and 2) major adverse cardiovascular events (MACE) defined as coronary artery bypass graft/percutaneous coronary intervention procedure, incident MI, mortality due to MI, incident stroke/transient ischemic attack, or mortality due to stroke.18 Secondary outcomes included all-cause hospitalizations and HF hospitalizations (measured as days hospitalized relative to days of follow-up, in each case). Data for hospitalizations were extracted from both VA data and Medicare to ensure capture of admissions both inside and outside VA. Mortality data were extracted from the National Death Index.19 Variable details are shown in Supplemental Table 1.
Covariates
Data were extracted from the VA clinical record, including age, race, sex, and body mass index; comorbidities including smoking status, anemia, arthritis, atrial fibrillation, cancer, chronic obstructive pulmonary disease, dementia, depression, diabetes, fatigue, gait issues, hyperlipidemia, hypertension, liver disease, renal disease, and sleep apnea. Risk of ASCVD was assessed using the VA ASCVD risk calculator.20 Frailty was measured using the VA Frailty Index.21,22 We removed stroke, coronary artery disease, and peripheral vascular disease from the VA Frailty Index calculation, since the study population excluded people with baseline ASCVD; and also removed HF from the VA Frailty Index calculation, since the study population only included adults with HF.
Information on other cardiovascular medications used including antiplatelet agents, anticoagulants, alpha-blockers, beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, calcium channel blockers, loop diuretics, and non-statin lipid-lowering medications were extracted from the VA clinical record and Centers for Medicare & Medicaid Services data. Veterans with use of medications from ≥5 drug classes were considered to have polypharmacy.
Analytic plan
To address confounding by indication,23 we developed a propensity score that included variables that can influence both the choice of using a statin and the outcome of interest, in order to balance covariates between users and nonusers of statins.24 We performed diagnostics on the propensity score to ensure adequate overlap between the exposed and unexposed groups using Kernel Density plots and assessing standard differences before and after running the propensity score. We used the overlap weighting method for the propensity score,25,26 and then used survival analysis to model time to each outcome.
To test the association between new statin use and the primary outcomes of all-cause mortality and MACE, we performed Cox proportional hazards regression models, using the overlap weight from the propensity modeling, and measured time to event. For all primary aim models, HRs and 95% CIs were calculated, overall and for subgroups of interest. We tested the proportional hazards assumptions for all models.
For the secondary outcomes of all-cause and HF-specific hospitalizations, we calculated the number of days hospitalized and ran Poisson regression models with the log of follow-up time as the offset (to model days hospitalized per year relative to total follow-up time), and calculated rate ratios (RRs) and 95% CIs. We assessed deviance statistics to check the goodness of fit of the Poisson models.
Stratified analyses for each outcome were conducted to test the interactions by age group (<50, 50-64, 65-74, and 75+), race, sex, diabetes history, ASCVD risk score (null, <5%, 5%-9.9%, and 10+%), and frailty (frailty score <0.20 vs ≥0.20). In sensitivity analysis, we further restricted the cohort to those who had at least 2 prescriptions for statins in follow-up. A 2-sided P value <0.05 was considered statistically significant. The study was approved by the VA Boston Healthcare System Institutional Review Board.
Results
The final curated study population included 7,970 Veterans with HFpEF who were free of ASCVD and prior statin use (Supplemental Figure 1). Among these, 3,759 (47%) initiated a statin over the follow-up period. At baseline, average age was 69 ± 12 years, 96% were male, 67% were White, 14% were Black, and mean ejection fraction was 60%. The most commonly prescribed statin was simvastatin (76%) followed by lovastatin (9%), atorvastatin (7%), and pravastatin (6%), all other statins were <1%. In total, 93.4% had 2 or more prescriptions over follow-up, representing at least 6 months of statin use. Before propensity score weighting, statin initiators were more likely to be younger, have metabolic syndrome, arthritis, and other chronic health conditions such as chronic kidney disease and sleep apnea. Non-statin users were older and more likely to have liver disease. Kernel Density plots demonstrated sufficient overlap for analyses (Supplemental Figure 2). Table 1 and Supplemental Figure 3 show cohort characteristics at baseline which were all balanced after overlap weighting.
Table 1.
Baseline Characteristics of 7,970 Veterans With HFpEF, Before and After Propensity Score Inverse Overlap Weighting
| Crude |
After Propensity Score Weighting |
|||||
|---|---|---|---|---|---|---|
| No Statin (n = 7,970) | Statin Use (n = 3,759) | Standardized Difference | No Statin (n = 7,970) | Statin Use (n = 3,759) | Standardized Difference | |
| Age, y | 69.2 ± 12.3 | 67.3 ± 11.2 | 16.4 | 67.9 ± 6.1 | 67.9 ± 8.2 | - |
| Body mass index, kg/m2 | 33.0 ± 8.4 | 34.9 ± 8.3 | 21.8 | 34.3 ± 4.3 | 34.3 ± 5.9 | - |
| VA Frailty Index (% frail) | 24.1 | 28.7 | 10.6 | 26.7 | 26.7 | - |
| Sex | ||||||
| Men | 96.3 | 96.4 | 0.2 | 96.5 | 96.5 | - |
| Women | 3.7 | 3.6 | 0.2 | 3.5 | 3.5 | - |
| Race | ||||||
| White | 66.6 | 69.8 | 7.0 | 68.7 | 68.7 | - |
| Black/African American | 14.3 | 16.2 | 5.5 | 15.9 | 15.9 | - |
| Other | 19.2 | 13.9 | 14.1 | 15.5 | 15.5 | - |
| Hispanic ethnicity | 2.5 | 2.0 | 3.0 | 2.2 | 2.2 | - |
| Smoking status | ||||||
| Current | 15.4 | 20.7 | 13.8 | 18.8 | 18.8 | - |
| Former | 70.3 | 59.4 | 22.9 | 63.0 | 63.0 | - |
| Never | 14.3 | 19.9 | 14.8 | 18.2 | 18.2 | - |
| Comorbidities | ||||||
| Atrial fibrillation | 19.2 | 25.0 | 14.1 | 23.1 | 23.1 | - |
| Anemia | 16.6 | 22.2 | 14.3 | 19.3 | 19.3 | - |
| Arthritis | 33.2 | 42.6 | 19.6 | 38.8 | 38.8 | - |
| Cancer | 25.6 | 30.0 | 9.9 | 28.3 | 28.3 | - |
| COPD | 32.5 | 42.5 | 20.8 | 38.4 | 38.4 | - |
| Dementia | 5.2 | 5.3 | 0.4 | 5.0 | 5.0 | - |
| Depression | 19.9 | 28.5 | 20.4 | 25.0 | 25.0 | - |
| Diabetes | 26.9 | 36.0 | 19.7 | 33.9 | 33.9 | - |
| Fatigue | 4.9 | 7.9 | 12.4 | 6.1 | 6.1 | - |
| Gait issues | 5.2 | 8.0 | 11.2 | 6.3 | 6.3 | - |
| Hyperlipidemia | 17.1 | 47.7 | 69.4 | 34.1 | 34.1 | - |
| Hypertension | 64.9 | 85.6 | 49.3 | 80.2 | 80.2 | - |
| Kidney disease | 2.8 | 7.2 | 20.4 | 4.7 | 4.7 | - |
| Liver disease | 2.4 | 1.7 | 4.6 | 1.9 | 1.9 | - |
| Sleep apnea | 10.8 | 24.1 | 35.6 | 18.0 | 18.0 | - |
| Medication use | ||||||
| ACE inhibitors | 53.8 | 62.2 | 17.1 | 60.4 | 60.4 | - |
| ARB | 8.6 | 12.5 | 12.7 | 11.0 | 11.0 | - |
| Alpha blockers | 23.0 | 21.8 | 2.9 | 22.9 | 22.9 | - |
| Beta-blockers | 43.5 | 55.9 | 25.0 | 52.2 | 52.2 | - |
| Calcium-channel blockers | 32.7 | 36.6 | 8.2 | 35.9 | 35.9 | - |
| Diuretics | 97.3 | 91.3 | 26.2 | 95.9 | 95.9 | - |
| Anticoagulants | 13.5 | 16.0 | 7.1 | 15.4 | 15.4 | - |
| Antiplatelets | 1.5 | 6.2 | 24.7 | 3.1 | 3.1 | - |
| Non-statin lipid-lowering medications | 4.4 | 7.7 | 13.9 | 6.4 | 6.4 | - |
| Polypharmacy (5 or more medication classes) | 83.3 | 95.1 | 38.5 | 93.3 | 93.3 | - |
Values are mean ± SD or %.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blockers; COPD = chronic obstructive pulmonary disease; HFpEF = heart failure with preserved ejection fraction; VA = Veterans Affairs.
Primary outcome
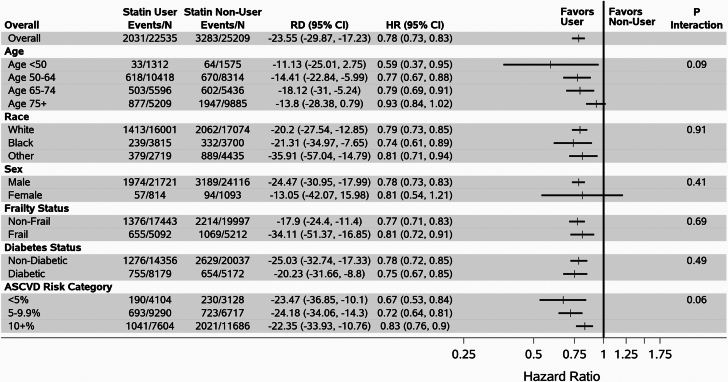
Over a mean follow-up of 6.0 ± 4.0 years from HFpEF diagnosis, 5,314 deaths and 4,859 major ASCVD events occurred. The weighted incidence rate difference per 1,000 person-years was −23.55 (95% CI: −29.87 to −17.23) for all-cause mortality and −46.33 (95% CI: −56.38 to −36.29) for MACE. After propensity score overlap weighting, the HR for statin users vs nonusers was 0.78 (95% CI: 0.73-0.83) for all-cause mortality, and 0.79 (95% CI: 0.74-0.84) for MACE (Table 2, Figure 1). Results were consistent among multiple subgroups (Figure 1). For all-cause mortality, there were no significant interactions after stratifying by age, including above age 75, race, sex, diabetes, frailty, or ASCVD risk score (all P > 0.05).
Table 2.
Association of Statin Use With All-Cause Mortality, Major Cardiovascular Events, and Hospitalizations in 7,970 U.S. Veterans With HFpEF and Free of ASCVD at Baseline, After Propensity Score Overlap Weighting
| Statin User N = 3,759 |
Statin Nonuser N = 7,970 |
Outcome | HR (95% CI) | P Value | |
|---|---|---|---|---|---|
| Primary outcome | # events/1,000 person-y | # events/1,000 person-y | Weighted incidence rate difference/1,000 person-y (95% CI) | ||
| All-cause mortality | 2,031/22.5 | 3,283/25.2 | −23.55 (−29.87, −17.23) | 0.78 (0.73-0.83) | <0.001 |
| MACE compositea | 1,576/13.9 | 3,283/18.8 | −46.33 (−56.38, −36.29) | 0.79 (0.74-0.84) | <0.001 |
| Secondary outcome | # days hospitalized/1,000 person-y | # days hospitalized/1,000 person-y | Risk difference (difference in # days hospitalized/y) | ||
| All-cause hospitalization days per y | 108.0/22.3 | 169.2/24.8 | −2.11 (−2.97, −1.26) | 0.69 (0.60-0.80) | <0.001 |
| HF-specific hospitalization days per y | 41.4/22.3 | 61.3/24.8 | −0.69 (−1.12, −0.26) | 0.72 (0.59-0.88) | <0.001 |
ASCVD = atherosclerotic cardiovascular disease; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; MACE composite = major adverse cardiovascular events composite.
MACE composite = MI/MI death, stroke/stroke death, revascularization.
Figure 1.
Association Between Statin Use and All-Cause Mortality in 7,970 U.S. Veterans With HFpEF Free of Atherosclerotic Cardiovascular Disease at Baseline, Stratified by Age, Race, Sex, Frailty, Diabetes, and ASCVD Risk Category
Events/N = # of deaths per # of person-years of follow-up; RD = weighted incidence rate difference (ie, the # of fewer deaths per 1,000 Person-years for statin users vs nonusers).
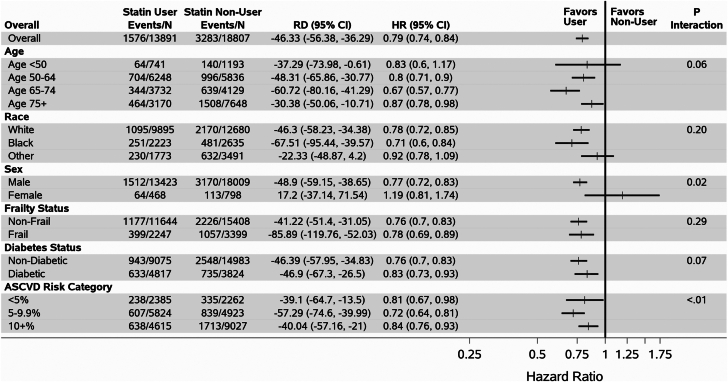
For MACE, results remained overall consistent; however, there were significant interactions by sex and ASCVD risk category; and a borderline-significant interaction by age (Figure 2). Among those aged 65 to 74 years, HR for MACE was 0.67 (95% CI: 0.57-0.77), while for those aged <50, 50 to 64 or ≥75 years HRs were 0.83 (95% CI: 0.60-1.17), 0.80 (95% CI: 0.71-0.90), and 0.87 (95% CI: 0.78-0.98), respectively, overall p-interaction 0.06 for age. For men, HR for MACE was 0.77 (95% CI: 0.72-0.83) and for women, HR was 1.19 (95% CI: 0.81-1.74), P value for interaction was 0.02. Among those with an ASCVD risk score 5% to 9.9%, HR for MACE was 0.72 (95% CI: 0.64-0.81), while the HR for those with an ASCVD <5% and >10% was 0.81 (95% CI: 0.67-0.98) and 0.84 (95% CI: 0.76-0.93), respectively, p-interaction was <0.01.
Figure 2.
Association Between Statin Use and Major Adverse Cardiovascular Events in 7,970 U.S. Veterans With HFpEF Free of Atherosclerotic Cardiovascular Disease at Baseline, Stratified by Age, Race, Sex, Frailty, Diabetes, and ASCVD Risk Category
Events/N = # of deaths per # of person-years of follow-up; RD = weighted incidence rate difference (ie, the # of fewer deaths per 1,000 Person-years for statin users vs nonusers).
Secondary outcomes
Over the course of the study period, there were 7,256 hospitalizations, including 4,632 HF-specific hospitalizations. RR for all-cause hospitalization was 0.69 (95% CI: 0.60-0.80) and for HF-specific hospitalization was 0.72 (95% CI: 0.59-0.88), Table 2. There were no significant interactions when stratified by age, race, sex, frailty status, diabetes history, or ASCVD risk category (Supplemental Figures 4 and 5).
Sensitivity analysis
When the cohort was restricted to those with 2 or more statin prescriptions during follow-up, results remained consistent for all outcomes (Supplemental Table 2).
Discussion
In this study of 7,970 Veterans with prevalent HFpEF, free of ASCVD at baseline, and who were regular users of VA care, nearly half were initiated on statin therapy over a mean follow-up of 6 years. New statin use was associated with a significant reduction in all-cause mortality, MACE, and both all-cause and HF hospitalizations (Central Illustration). This has important implications on a 2-decade search for a therapy to improve survival in HFpEF; in particular, it strengthens the foundation for a potential RCT to examine the effect of statins in HFpEF.
Central Illustration.
Association of New Statin Use With All-Cause Mortality, MACE, and Hospitalizations in Veterans HFpEF and No Atherosclerotic Cardiovascular Disease
ASCVD = atherosclerotic cardiovascular disease; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; MACE = major adverse cardiovascular events.
In the context of the current literature
Data remain limited on the utility of statins in HF. Two major RCTs, CORONA and GISSI-HF have been predominantly conducted in participants with HFrEF. In the CORONA trial, 5,011 individuals aged at a mean of 73 years with NYHA functional class II-IV HF had a mean left ventricular ejection fraction (LVEF) was 31%. With randomization to rosuvastatin 10 mg or placebo and follow-up over nearly 33 months, there was no significant benefit associated with statin therapy in prevention of the composite outcome of cardiovascular death, nonfatal MI, or fatal stroke and secondary outcomes of CVD mortality and any coronary events.6 The GISSI-HF trial, which included 4,575 participants with a mean age of 68 years, did not require a specific LVEF threshold, but ultimately enrolled mostly participants with HFrEF (mean LVEF of 33%). The initiation of rosuvastatin 10 mg over a follow-up of 47 months demonstrated no significant difference in the endpoints of all-cause mortality or the composite of mortality and hospitalization from CVD causes in both ischemic and nonischemic HF when compared to placebo. Despite inclusion of any LVEF, a major limitation of this study was that a total of 461 participants had an LVEF >40% precluding meaningful inferences regarding statins in patients with HFpEF.7
To date, no RCTs have investigated the potential benefit of statins in HFpEF. However, there have been several observational studies. In a meta-analysis of 11 observational studies that included 17,985 patients with HFpEF, statin use was associated with a decreased risk of mortality (RR: 0.60; 95% CI: 0.49-0.74; P < 0.001).11 Another meta-analysis that included only studies that employed propensity score methods to address confounding by indication (4 observational studies; n = 5,536) also reported a mortality benefit among statin users (OR: 0.69; 95% CI: 0.49-0.97; P = 0.030).12 However, these studies were not restricted to primary prevention of ASCVD (42%-64% had underlying coronary artery disease); 2 had a sample size of <200 patients;13,27 and the majority of patients studied were from 2 European countries, Poland and Sweden.28,29 In addition to examining endpoints beyond ASCVD, including mortality and both HF and all-cause hospitalizations, our study extends the literature by investigating the benefit of statins for preventing primary MACE. Furthermore, the mean follow-up time for our study at 6 years was longer than the majority of studies included in both meta-analyses.
Our results are comparable to the propensity-adjusted cohort study that included 24,598 naive patients with newly diagnosed HF.30 Among those who initiated a statin, there was a 24% reduction in risk of all-cause mortality and 21% reduction in HF hospitalization compared to non-statin users. However, 80% of those studied had prior coronary heart disease, and while 29% of the statin users and 22% of the non-statin users had HFpEF, analyses were not stratified by type of HF. Additionally, this study demonstrated that event rates remained significantly lower in subgroups stratified by the presence or absence of baseline coronary heart disease. The analyses conducted in our study are novel in that they focus specifically on a cohort with HFpEF for primary prevention of CVD, and the methods employed in our study uniquely consist of both a new user study design and propensity score methods to address confounding by indication.
Potential mechanisms
The mechanisms for which statins may be beneficial in HFpEF are not yet clear. Our findings were among adults without baseline ASCVD and were consistent across pooled-cohort, 10-year risk categories. This suggests that the benefits of statins for HFpEF go beyond its impact on atherosclerosis. Statins have multiple biological effects—although they are principally used as cholesterol-lowering agents,31 they also have pleiotropic effects with capacity to reduce inflammation.32 This is relevant for HFpEF because its pathophysiology is closely linked to systemic inflammation,33 with consequent derangements in microvasculature, nitric oxide and cyclic guanosine monophosphate availability, and changes in titin phosphorylation.34 Based on myocardial biopsies from individuals with HFpEF, statins lower levels of myocardial nitrotyrosine (a marker of oxidant stress), reduce cardiomyocyte hypertrophy and resting tension, and increase myocardial protein kinase G activity.10,34 Statins are also known to lower risk of atrial fibrillation35 which leads to remodeling of the left atrium and is an important pathophysiologic feature of HFpEF.35,36 This could represent another etiologic mechanism.
It is also possible that the impact of statins is mediated through physical parameters such as frailty, which is highly prevalent in HFpEF and is closely intertwined with the pathophysiology of HFpEF.37 Taken together, our work here further supports the need for additional mechanistic studies that include detailed cardiovascular imaging data and biomarkers, as well as extracardiac markers of aging such as physical frailty. We additionally call for a trial specifically examining the effect of statins in adults with HFpEF without prior ASCVD. Of note, statins have a unique mechanism of action compared to other guideline-directed agents for HFpEF, most of which were not in routine use for HFpEF during the study period. This provides additional rationale for statins to serve as add-on therapy for patients with HFpEF, though more evidence is needed.
Clinical implications and future directions
The current joint society guidelines on the management of HF recommend statins in patients with a history of prior or recent MI to prevent symptomatic HF and adverse CVD events.4 Notably, in prevalent HF, there are no specific recommendations for the primary prevention of future adverse events within the current guidelines. This is largely based on the neutral findings of the CORONA and GISSI-HF trials for HFrEF. However, the lack of RCTs testing statins in HFpEF precludes any meaningful recommendations for this unique HF phenotype. Our study has important implications on a 2-decade search for a therapy to improve survival in HFpEF; in particular, it lays the groundwork for trials to examine the benefits of statins in HFpEF, which may shape future clinical practice.
Study Strengths and limitations
This study has several strengths. This is a highly curated clinical data set that combines both electronic health record data and claims data, with the inclusions of all recorded LVEFs, to ensure close to complete capture of data. We restricted to those without a history of ASCVD to focus on primary prevention as there is less debate about the use of statins for secondary prevention. A new user design ensured everyone in the cohort had the same probability of receiving a statin and to reduce the risk of survivor and prevalent user biases.38 Propensity overlap weighting addressed confounding by indication and limited to influence of extreme propensity scores.
This study also has important limitations. The data were restricted to users of VA which may limit generalization to populations not receiving care in the VA. The predominance of the cohort is male, although most prior studies have been mostly female, and this work therefore provides insight for men with HFpEF who are understudied. The most commonly prescribed statin was simvastatin, which no longer reflects current practice. Additionally, the MACE analysis may be impacted by competing risk for mortality, however although the CV specific mortality was included in the MACE definition this represents the majority of mortality. Although we used several approaches to mitigate confounding, this observational study is inherently limited by residual confounding and potential for reverse causation.
Conclusions
Among Veterans with HFpEF without known CVD, statins were associated with reduced all-cause mortality and MACE. These findings should be confirmed in a future RCT.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Statins, a readily accessible and highly effective class of lipid-lowering therapy for primary prevention of ASCVD, may lower the risk of adverse outcomes in adults who have HFpEF without concurrent ASCVD.
TRANSLATIONAL OUTLOOK: Statins could be considered for prevention of mortality, MACE, and hospitalization in patients with HFpEF. Future RCTs are needed to support these findings.
Funding support and author disclosures
This research was supported by an investigator-initiated grant from Otsuka Pharmaceuticals (Dr Joseph), VA CSR&D CDA-2 award IK2-CX001800 (Dr Orkaby), and National Institute on Aging R03-AG060169 (Dr Orkaby). Drs Djousse and Gaziano have received research funding from Novartis Pharmaceuticals, unrelated to this study. This paper does not represent the views of the Department of Veterans Affairs or the US government. Support for VA/Centers for Medicare & Medicaid Services data is provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (project numbers SDR 02-237 and 98-004). The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Contributor Information
Ariela R. Orkaby, Email: aorkaby@bwh.harvard.edu.
Jacob Joseph, Email: Jacob.joseph@va.gov.
Supplementary data
References
- 1.Baigent C., Blackwell L., Emberson J., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone N.J., Robinson J.G., Lichtenstein A.H., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K., Grossman D.C., Curry S.J., et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997–2007. doi: 10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 5.van der Harst P., de Boer R.A. Statins in the treatment of heart failure. Circ Heart Fail. 2010;3:462–464. doi: 10.1161/CIRCHEARTFAILURE.110.956342. [DOI] [PubMed] [Google Scholar]
- 6.Kjekshus J., Apetrei E., Barrios V., et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 7.Tavazzi L., Maggioni A.P., Marchioli R., et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 8.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 10.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Liu G., Zheng X.X., Xu Y.L., Ru J., Hui R.T., Huang X.H. Meta-analysis of the effect of statins on mortality in patients with preserved ejection fraction. Am J Cardiol. 2014;113:1198–1204. doi: 10.1016/j.amjcard.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Fukuta H., Goto T., Wakami K., Ohte N. The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol. 2016;214:301–306. doi: 10.1016/j.ijcard.2016.03.186. [DOI] [PubMed] [Google Scholar]
- 13.Fukuta H., Sane D.C., Brucks S., Little W.C. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 14.Patel Y.R., Robbins J.M., Kurgansky K.E., et al. Development and validation of a heart failure with preserved ejection fraction cohort using electronic medical records. BMC Cardiovasc Disord. 2018;18:128. doi: 10.1186/s12872-018-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel Y.R., Kurgansky K.E., Imran T.F., et al. Prognostic significance of baseline serum sodium in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund J.L., Richardson D.B., Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–228. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orkaby A.R., Cho K., Cormack J., Gagnon D.R., Driver J.A. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology. 2017;89(18):1877–1885. doi: 10.1212/WNL.0000000000004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floyd J.S., Blondon M., Moore K.P., Boyko E.J., Smith N.L. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol Drug Saf. 2016;25:467–471. doi: 10.1002/pds.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center of Excellence for Suicide Prevention Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository – National Death Index (NDI) http://vaww.virec.research.va.gov/Mortality/Overview.htm
- 20.Vassy J.L., Lu B., Ho Y.-L., et al. Estimation of atherosclerotic cardiovascular disease risk among patients in the Veterans Affairs Health Care System. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orkaby A.R., Nussbaum L., Ho Y.L., et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2018;74(8):1257–1264. doi: 10.1093/gerona/gly232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrauner W., Lord E.M., Nguyen X.T., et al. Frailty and cardiovascular mortality in more than 3 million US Veterans. Eur Heart J. 2021;43(8):818–826. doi: 10.1093/eurheartj/ehab850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeger J.D., Kurth T., Walker A.M. Use of propensity score technique to account for exposure-related covariates: an example and lesson. Med Care. 2007;45:S143–S148. doi: 10.1097/MLR.0b013e318074ce79. [DOI] [PubMed] [Google Scholar]
- 24.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeger J.D., Bykov K., Bartels D.B., Huybrechts K., Schneeweiss S. Propensity score weighting compared to matching in a study of Dabigatran and Warfarin. Drug Saf. 2017;40:169–181. doi: 10.1007/s40264-016-0480-3. [DOI] [PubMed] [Google Scholar]
- 26.Elze M.C., Gregson J., Baber U., et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69:345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Roik M., Starczewska M.H., Huczek Z., Kochanowski J., Opolski G. Statin therapy and mortality among patients hospitalized with heart failure and preserved left ventricular function--a preliminary report. Acta Cardiol. 2008;63:683–692. doi: 10.2143/AC.63.6.2033384. [DOI] [PubMed] [Google Scholar]
- 28.Nochioka K., Sakata Y., Miyata S., et al. Prognostic impact of statin use in patients with heart failure and preserved ejection fraction. Circ J. 2015;79:574–582. doi: 10.1253/circj.CJ-14-0865. [DOI] [PubMed] [Google Scholar]
- 29.Alehagen U., Benson L., Edner M., Dahlström U., Lund L.H. Association between use of statins and mortality in patients with heart failure and ejection fraction of ≥50. Circ Heart Fail. 2015;8:862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 30.Go A.S., Lee W.Y., Yang J., Lo J.C., Gurwitz J.H. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 31.Adhyaru B.B., Jacobson T.A. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15:757–769. doi: 10.1038/s41569-018-0098-5. [DOI] [PubMed] [Google Scholar]
- 32.Liao J.K., Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy S.P., Kakkar R., McCarthy C.P., Januzzi J.L. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Lam C.S.P., Voors A.A., de Boer R.A., Solomon S.D., van Veldhuisen D.J. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018;39:2780–2792. doi: 10.1093/eurheartj/ehy301. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.C., Voskoboinik A., Gerche A., Marwick T.H., McMullen J.R. Prevention of pathological atrial remodeling and atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:2846–2864. doi: 10.1016/j.jacc.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Santhanakrishnan R., Wang N., Larson M.G., et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved vs reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey A., Kitzman D., Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019;7:1001–1011. doi: 10.1016/j.jchf.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray W.A. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.