ABSTRACT
Background:
Aortico right atrial tunnel (ARAT) is a rare extracardiac communication between the aorta and the right atrium with two anatomical types. A recent global review identified 59 patients.
Methods:
Patients with ARAT from two centers were analyzed for their demographics, symptoms, morphology, management, and follow-up thromboprophylaxis.
Results:
Among 21 patients including 8 males with a median age of 3 years (18 days–72 years) diagnosed as ARAT, 12 (57%) had posterior tunnels and 9 had anterior tunnels. Four patients had multiple exits. Eighteen tunnels were closed after arteriovenous circuit formation. Six patients (29%) weighing <10 kg presented early with heart failure. Transcatheter closure normalized the hemodynamics including in one infant after failed surgery. Two elderly patients (10%) above 60 years presented with angina and atrial fibrillation. The rest were asymptomatic. Occluders were positioned in the narrow proximal aortic end of the tunnel in all except two patients, where the distal atrial end was closed. All procedures were successful without complications. There was one late death after 1 year from subarachnoid hemorrhage. At a median follow-up of 96 months, all survivors were asymptomatic. Thromboprophylaxis with dual antiplatelets for 1–2 years followed earlier was recently changed to aspirin with Coumadin. Complete remodeling occurred when the proximal aortic end was closed, but partial persistence of the track was noted after distal closure.
Conclusions:
This largest cohort of ARAT showed the safety and efficacy of transcatheter closure even in neonates. The narrow proximal aortic end should be the target for closure rather than the distal atrial end to achieve complete remodeling.
Keywords: Aorto-cardiac connection, coronary artery fistula, left-to-right shunt, nitinol occluder devices, obligatory shunt, remodeling
INTRODUCTION
Aorta-to-right atrial tunnel is a rare congenital anomaly characterized by an extracardiac connection between the aortic root and the right atrium.[1] Its last part before termination into the right atrium is always intracardiac within the atrium. Even though the first case described in 1980 was originating from the noncoronary sinus, all the subsequent published cases could be grouped into one of the two common distinct anatomical types.[2] They were the posterior tunnels from the left aortic sinus coursing posterior to the aortic root and the anterior tunnels from the anterior right aortic sinus coursing to the right of the aortic root.[3] Being an obligatory shunt with high-pressure gradients, it can be detected in fetal echocardiography and may lead to neonatal heart failure.[4,5] In spite of the presence of a continuous murmur, it may be surprisingly missed until adult life when patients present with heart failure, arrhythmia, or endocarditis.[6,7,8]
Most of the published case series reported only a small number of patients. A recent major attempt extracted all previous publications spanning 41 years across the world from 1980 to 2021 and enrolled 59 patients with this rare anomaly.[9] Conventional surgery closed either the aortic or the atrial end or both on cardiopulmonary bypass, though ligation of the tunnel on a beating heart could also arrest the shunt.[3,10,11] Transcatheter management involved antegrade or retrograde closure using coils, vascular plugs, or off-label use of duct occluders.[12,13] This report details the clinical profile, imaging, management, and follow-up of a large cohort of patients from two institutions.
METHODS
A retrospective analysis of the case records of patients who were diagnosed to have aortico right atrial tunnels (ARATs) in the past 15 years was approved by the institutional review board from two tertiary care hospitals. Consent from patients for this analysis was waived as the report was anonymized. Demographic data collected included age, sex, weight, symptoms, clinical findings, features of heart failure, cardiomegaly on chest X-ray, and the underlying cardiac rhythm. A transthoracic echocardiogram identified the type of the tunnel, its course, dimensions, the presence of pulmonary arterial hypertension, and ventricular dysfunction. Indications of closure of the tunnel were either symptoms of heart failure in young patients or angina secondary to steal in older patients. Asymptomatic patients with large tunnels were closed after they reached more than 10 kg body weight if the tunnels were larger than 10 mm.
Anatomy of the tunnel
The anatomy of the two types of ARATs was surprisingly uniform and stereotyped with a predictable pattern of their origin and course.[9] Anterior tunnels always arose from the right aortic sinus in close relation to the right coronary artery origin and coursed on the right side of the aortic root to terminate in the right atrium [Figure 1]. The more common posterior tunnels always arose from the left sinus close to the left main coronary artery origin and coursed behind the aortic root toward the right atrium [Figure 2]. Both these tunnels often had a narrower proximal segment in close vicinity of the aortic root compared to a much wider, often aneurysmal distal segment before its termination in the right atrium. The tunnels were differentiated from coronary cameral fistula by lack of any myocardial branches, sharp U-turn near the aortic origin, and normal size of the ipsilateral coronary artery.[9]
Figure 1.
Anterior aortico atrial tunnel (a) is seen in the parasternal short axis originating from the anterior aortic sinus and courses to the right making a sharp U-turn before terminating in the right atrium (RA) with an aneurysmal segment (T). Subxiphoid view (b) with color Doppler (c) shows the tunnel in the atrial septum between the RA and the left atrium. The aneurysmal tunnel is also shown in the parasternal short axis (d and e) and apical view (f). LCA: Left coronary artery; RCA: Right coronary artery
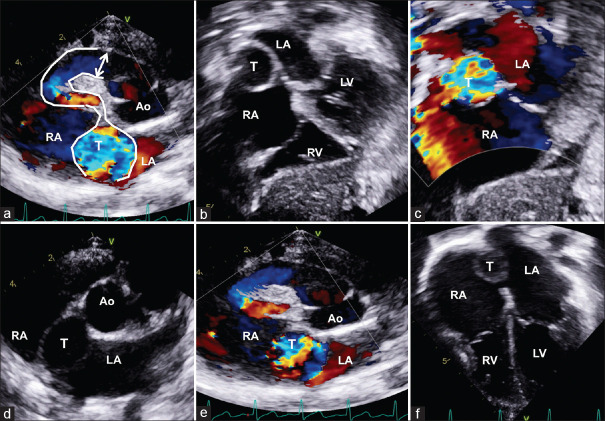
Figure 2.
Transesophageal echocardiogram (a) of posterior aortico right atrial tunnel (T). The T originates from the aortic (Ao) left sinus, turns posteriorly behind the Ao root and enters the right atrium (RA). In its course, it passes through the interatrial septum (b) between the left atrium (LA) and RA. Transthoracic echocardiogram (c) with color Doppler in apical five-chamber view shows the extracardiac portion of aorto–right atrial T passing across the LA and opening into RA with mosaic pattern. Short axis with color Doppler (d) shows the T covering from the left side opening into RA with turbulence. LCA: Left coronary artery; RCA: Right coronary artery
Preprocedural imaging
As the anatomy was often stereotypic, additional imaging with transesophageal echocardiogram or computed tomographic angiography was seldom employed for further delineation of the anatomy. Posterior tunnels were best imaged on aortic root angiography performed in an anteroposterior projection with a steep caudal angulation. The narrowest proximal portion would often be below and behind the aortic root which was usually the ideal target for the occluder placement [Figure 3]. On the contrary, the entire length of the anterior tunnels was visualized well on a right anterior oblique projection. In these patients, the narrowest proximal part would overlap the aortic root in this view and would be the target zone for occluder placement.
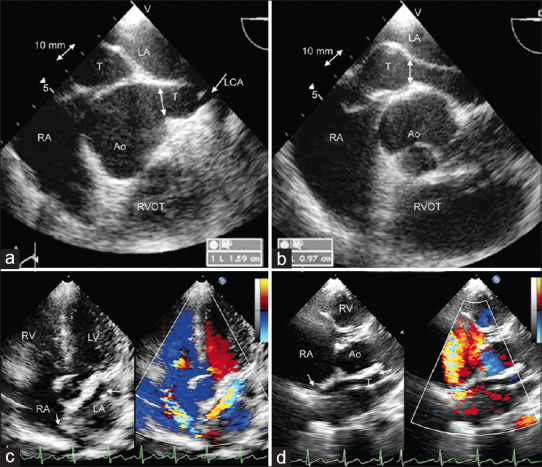
Figure 3.
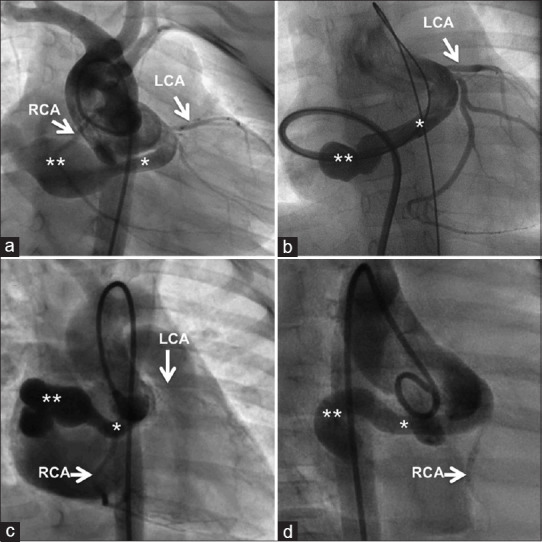
The anatomy of the posterior aortico right atrial tunnel from the left aortic sinus is best defined by an angiogram from the aortic root (a) or through a sheath advanced from the atrial end (b) in anteroposterior view with caudal projection. The anterior tunnel from the right aortic sinus is best delineated from aortic root angiogram (c and d) in the right anterior oblique projection. All tunnels uniformly have a narrow proximal segment close to the aortic root (*) and a distal aneurysmal segment (**) before its termination. LCA: Left coronary artery; RCA: Right coronary artery
Preparation for interventional closure
After administering a single preprocedural antiplatelet dose of aspirin, the procedure was done under conscious sedation and local anesthesia and obtaining a femoral vein and arterial access. Intubation anesthesia was limited to hemodynamically compromised neonates and young infants. Following hemodynamic pressure recordings, an aortic root angiogram was performed in the views mentioned above. Selective coronary angiogram was done in adults to exclude atherosclerotic stenosis. Oximetric shunt quantification was not mandatory as the obligatory shunt was clinically evident with cardiomegaly and chamber enlargement.
Closure of the tunnel after formation of arteriovenous circuit
The tunnel was crossed from the aortic side using an angled hydrophilic GLIDEWIRE (Terumo Corporation, Tokyo, Japan), and the wire tip was snared from a venous catheter to establish an arteriovenous circuit. An appropriate-sized long Mullins sheath (Cook Medical, Bloomington, IN) or a coronary guide catheter was advanced from the venous end, deep into the tunnel to reach its aortic end. An adequate-sized duct occluder device or a vascular plug was deployed very proximally immediately after the coronary artery origin to reduce the length of the cul-de-sac in the tunnel [Figure 4]. When vascular plugs were not available in the earlier period of the study, multiple Gianturco coils (Cook Medical, Bloomington, IN) were delivered together aided by bioptome forceps [Figure 5]. In a few cases that showed a persistent flow through the initial occluder placement, a second duct occluder or 1–2 coils were deployed near the right atrial exit of the tunnel [Figure 6].
Figure 4.
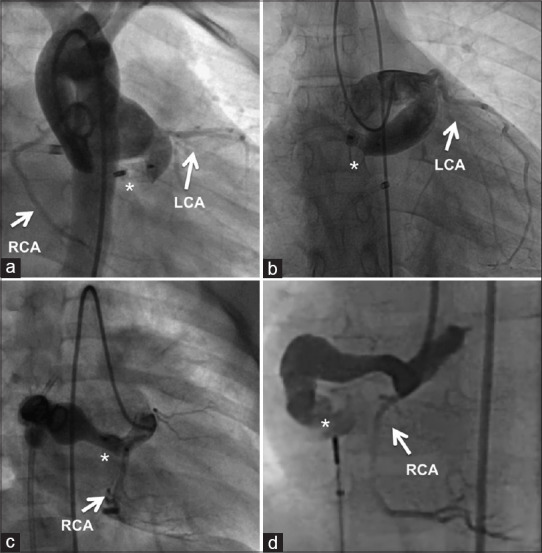
The ideal location for closure in the posterior tunnel is the proximal narrow segment (a), where an occluder is deployed leaving a very short cul-de-sac. A more distal placement of the occluder leaves behind a longer cul-de-sac in posterior tunnels (b). Anterior tunnel (c) is also ideally closed in the proximal segment by an occluder. Distal occluder placement in the anterior tunnel (d) leaves a very long residual track of the tunnel. The occluder location is shown by (*). LCA: Left coronary artery; RCA: Right coronary artery
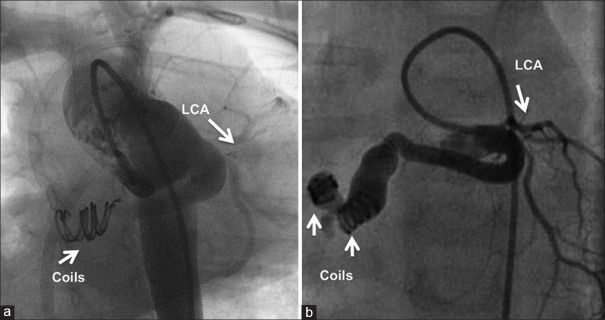
Figure 5.
The posterior tunnel is closed by multiple coils aided by the bioptome (a) delivered from the atrial end of the tunnel. If an arteriovenous circuit is not feasible, coils are delivered antegrade from the aortic end (b). LCA: Left coronary artery; RCA: Right coronary artery
Figure 6.
Anterior tunnel is delineated in lateral view (a), which also shows the right coronary artery. It is closed by Amplatzer Vascular Plug IV (b) through an arterial diagnostic catheter
Closure without arteriovenous circuit formation
When the formation of an arteriovenous circuit led to hemodynamic or electrocardiographic disturbances in very small patients, retrograde delivery of coils was performed through a 4F diagnostic catheter. Alternatively, low-profile devices such as Amplatzer vascular plug IV (Abbott, Plymouth, MN) had been retrogradely delivered through a similar diagnostic arterial catheter in recent times [Figure 7]. The target zone for the device placement was typically in the narrow segment immediately beyond the coronary artery origin. A final angiogram confirmed the position of the occluder, residual shunt, and unobstructed coronary flows.
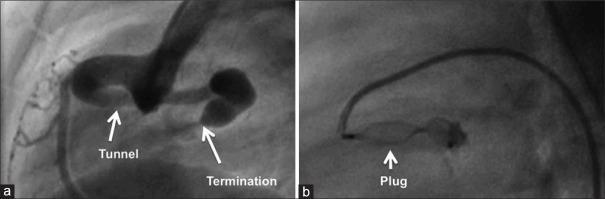
Figure 7.
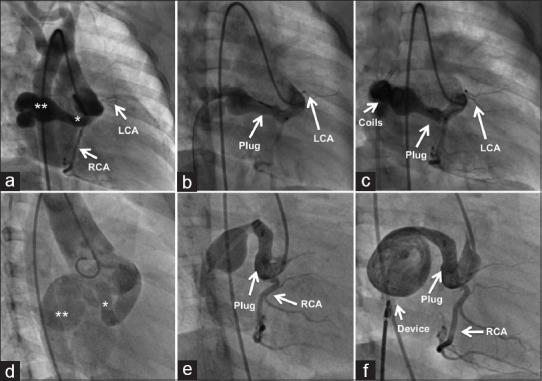
After delineating a narrow proximal end (*) and an aneurysmal distal end (**) of the anterior tunnel in right anterior oblique projection (a), the proximal end is closed with a vascular plug (b) and the distal aneurysmal end is closed with multiple coils (c). Another anterior tunnel (d) is closed similarly in the proximal narrow end (*) by the vascular plug (e) and the atrial exit beyond the aneurysmal segment is closed with a duct occluder (f). LCA: Left coronary artery
Postprocedural follow-up
The antiplatelet–anticoagulation protocol had changed over the study period. While aspirin was administered alone 15 years ago, clopidogrel was added to aspirin in the last decade. Thromboprophylaxis was recently escalated to aspirin and Coumadin to maintain an international normalized ratio of 2.0–2.5. Imaging on follow-up was performed with echocardiography to identify residual flow, remodeling of the dilated segments, and exclude thrombus in the cul-de-sac before downscaling the anticoagulation regimen. A conventional catheter angiogram was performed in consenting patients to study the remodeling. Complete remodeling was defined as a total obliteration of the tunnel with normal blood flows in the coronary arteries [Figure 8]. Partial remodeling was defined as persistent filling of the track of the tunnel, even though there were no residual flows [Figure 9].
Figure 8.
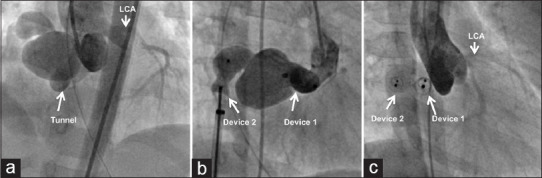
Anterior tunnel shown in aortic root angiogram (a) is closed with a first duct occluder in the proximal end and a second occluder in the atrial exit (b). Complete remodeling (c) is shown in a repeat angiogram after 2 years. LCA: Left coronary artery; RCA: Right coronary artery
Figure 9.
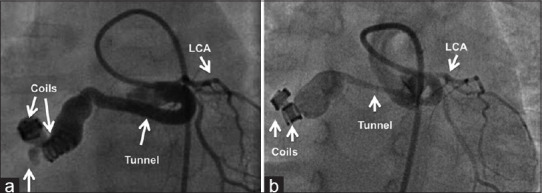
Posterior tunnel closed with two coils delivered from the arterial end (a) is shown to have partial remodeling (b) with persistence of the track in a repeat angiogram after 18 months. LCA: Left coronary artery
RESULTS
Twenty-one patients (8 males) with a median age of 3 years, ranging from 18 days to 72 years, were diagnosed to have ARAT in the two institutions between 2009 and 2023. The median weight of the cohort was 14 kg and ranged from 3 to 90 kg. Table 1 summarizes the patient’s details.
Table 1.
Patient details
| Age/sex | Weight (kg) | Type | Symptoms | Shunt ratio | Route | Device | Site of closure | Residual | Drugs | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3/female | 14.9 | Post | - | 1.5 | Retro | 8–6 MFO | Aortic end | None | Warf, asp | 18 |
| 23/female | 56 | Ant | - | 1.9 | Ante | 14 AVP2 | Aortic end | None | Clop, asp | 66 |
| 4/female | 13 | Post | - | - | Ante | 6–4 Cera | Aortic end | None | Clop, asp | 21 |
| 3/male | 14 | Ant | - | Ante | 5–4 ADO2 coils | Aortic and terminal end | None | Clop, asp | 72 | |
| 1/female | 8 | Post | CHF, PAH | 1.5 | Ante | 8 AVP4 | Aortic end | Warf, asp | 12 | |
| 0.7/male | 3 | Post | CHF, PAH | Ante | 12 AVP2 coil | Aortic and terminal end | None | Clop, asp | 144 | |
| 1/female | 8 | Ant | CHF, PAH | Ante | 5–4 Piccolo | Aortic end | None | Clop, asp | 18 | |
| 2/male | 11 | Ant | - | 6–4 Cera | Aortic end | None | Clop, asp | 42 | ||
| 19/male | 58 | Ant | - | 1.7 | 14–12 Cera | Aortic end | None | Clop, asp | 48 | |
| 1/female | 10 | Ant | CHF, PAH | Retro | 8 AVP4 | Aortic end | None | Warf, asp | 6 | |
| 5/female | 15 | Post | - | Ante | 12 AVP2 | Aortic end | None | Clop, asp | 42 | |
| 1/female | 10 | Ant | - | Ante | 10–8 Cera 8–6 Cera | Aortic and terminal end | None | Clop, asp | 120 | |
| 71/female | 76 | Post | AF, angina | Ante | 20–18 Cera | Terminal end | None | Warf, asp | 168 | |
| 0.1/female | 3 | Post | CHF, PAH | Ante | 8 AVP2 | Aortic end | None | Warf, asp | 12 | |
| 65/male | 90 | Post | AF, angina | Ante | 18 AVP2 | Aortic end | None | Clop, asp | 96 | |
| 7/female | 20 | Ant | - | Ante | 8 Cera plug 8–6 Cera | Aortic and terminal end | None | Clop, asp | 48 | |
| 25/male | 65 | Post | - | Ante | Coils | Aortic end | None | Clop, asp | 144 | |
| 10/male | 30 | Post | - | Ante | 8–6 Cera | Aortic end | None | Asp | 132 | |
| 0.7/female | 5 | Post | CHF, PAH | Retro | Coils | Mid portion | None | Asp | 132 | |
| 12/female | 40 | Ant | - | Ante | 12 AVP2 | Aortic end | None | Clop, asp | 84 | |
| 3/male | 12 | Post | - | Ante | 10 AVP2 | Aortic end | None | Warf, asp | 12 |
Ant: Anterior aortico right atrial tunnel, Post: Posterior aortico right atrial tunnel, AVP: Amplatzer vascular plug, ADO: Amplatzer duct occlude, Cera: Cera duct occlude, MFO: Konar multifunction occlude, Warf: Warfarin, asp: Aspirin, CHF: Congestive heart failure, PAH: Pulmonary hypertension, AF: Atrial fibrillation
Echocardiographic findings
Twelve out of 21 patients had a posterior tunnel originating from the left aortic sinus. Nine patients had an anterior tunnel. There were no tunnels from the noncoronary sinus. Two patients had more than one exit orifice in the right atrium [Figure 7]. The most dilated portion near the atrial end measured between 10 and 22 mm. Associated malformations included patent arterial duct in one patient who underwent concomitant device closure and small hemodynamically insignificant oval fossa defect in two patients. The left ventricular systolic function was normal in all patients, despite its enlargement. None of the patients had computed tomographic or magnetic resonance imaging.
Catheterization data
Patients weighing under 10 kg consisting primarily of infants had varying degrees of pulmonary hypertension. Two patients had near systemic pulmonary artery pressures. Eighteen procedures were completed from the venous end after achieving an arteriovenous loop, whereas the others were closed from the aortic end. The occluders were positioned in the narrow proximal segment in all except one patient. Coils placed in the proximal segment in one patient immediately migrated to the midportion of the tunnel after release. The tunnel in the first patient was closed at the atrial exit with a duct occluder in 2009 leaving a very long aneurysmal cul-de-sac. The occluders used included Cera duct occluder (Lifetech Scientific, Shenzhen, PRC) in six patients, Cera vascular plug (Lifetech Scientific, Shenzhen, PRC) in one patient, Amplatzer Duct Occluder II (Abbott, Plymouth, MN) in one patient, Piccolo device (Abbott, Plymouth, MN) in one patient, multiple coils aided with bioptome cup forceps in one patient, and Amplatzer Vascular Plug II (Abbott, Plymouth, MN) in the remaining eight patients. The exit at the aneurysmal atrial end was closed using an additional second device or coils in four patients. In three patients, direct closure was achieved from the aortic end through an arterial diagnostic catheter. The transaortic closure was done using the Konar MF device (Lifetech Scientific, Shenzhen, PRC) in the first patient, Amplatzer Vascular Plug IV in the second, and two embolization coils in the third patient. Closure of the tunnel was successful in all patients.
Usual clinical presentation
Incidental detection of a continuous murmur on auscultation during a routine examination was the most common clinical presentation in 13 patients. These asymptomatic patients were aged between 2 and 21 years. Closure of the tunnel was successful in all patients. Apart from minimal cardiomegaly, wide pulse pressure, and a continuous murmur, the clinical examination was unremarkable in these patients. Cardiac catheterization showed normal pulmonary artery pressures in all these patients. The pulmonary-to-systemic shunt ratio assessed by oximetry in four patients ranged from 1.5 to 1.9. Oximetry evaluation was not done in others as the obligatory shunt was clinically evident. They underwent elective device closure of the tunnel.
Very early clinical presentation at a young age
Six young patients weighing < 10 kg (median: 6 kg and range: 3–10 kg) presented early with features of heart failure, growth failure, or recurrent respiratory illnesses. The clinical features uniformly suggested a very large aortic runoff with bounding pulses, high pulse pressures, cardiomegaly, and loud continuous murmurs. One of these patients was diagnosed to have an anterior tunnel in fetal echocardiography and showed progressive rapid dilatation on serial postnatal imaging. Device closure of this tunnel at 1 and ½ years of age was reported earlier.[4] One infant aged 7 months with congestive heart failure and severe pulmonary hypertension underwent surgery through midline sternotomy without cardiopulmonary bypass. Closure of the tunnel was done by the placement of external clips over the track visualized after opening the pericardium in the transverse sinus. Postoperative echocardiogram after 24 h showed large persistent flows through the tunnel. This patient underwent catheter angiography after 2 weeks, which demonstrated that the clips were not occluding the tunnel. The tunnel was closed in the catheterization laboratory using a 12-mm Amplatzer Vascular Plug II in the proximal narrow segment and additional two embolization coils in the aneurysmal distal segment [Figure 10]. The youngest patient was a neonate aged 18 days who presented with severe pulmonary hypertension and respiratory distress and was on mechanical ventilatory support. The infant underwent closure with an 8-mm Amplatzer Vascular Plug II after arteriovenous loop formation.
Figure 10.
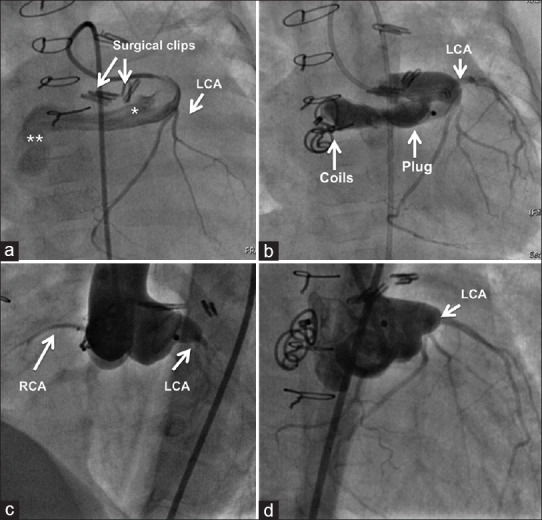
Failure to close a posterior tunnel after attempted surgery by placing surgical clips over the track (a) is dealt by placing vascular plug (b) in the narrow proximal segment (*) and coils in the distal aneurysmal segment (**). Aortogram after three years shows a near complete remodeling in left anterior oblique (c) and right anterior oblique (d) projections
Clinical presentation in elderly patients
Two adults aged 65 and 71 years presented with effort angina. Both these patients had atrial fibrillation and cardiomegaly on clinical examination but did not show any continuous murmur on auscultation. The echocardiogram showed a large tunnel with a continuous color Doppler flows from the left aortic sinus coursing posterior to the aortic root with termination in the right atrium. Fluoroscopy demonstrated a faint rim of calcification in the course of the tunnel. Selective coronary angiography did not reveal any atherosclerotic narrowing. After interventional closure, attempts to electrically cardiovert atrial fibrillation failed despite pretreatment with amiodarone. Refractoriness to cardioversion was presumed to be due to altered intra-atrial electrical conduction caused by the regions of calcifications in the course of the tunnel. They remained free of anginal pain and continued to receive Coumadin on follow-up.
Follow-up
There were no procedural complications at a median follow-up of 96 months (90 ± 56 months). The electrocardiogram did not show any changes of myocardial ischemia following the intervention. An echocardiogram showed the lack of residual flow in all patients as well as the absence of regional wall motion abnormalities. Thromboprophylaxis was discontinued after 1–2 years in all patients except the two elderly patients who were receiving Coumadin for atrial fibrillation.
Death on follow-up
There was one death on follow-up. The 18-day-old neonate who underwent closure of the tunnel for refractory heart failure and respiratory failure improved significantly with normal growth and development. She received aspirin and clopidogrel on follow-up. An elective check angiogram was performed at 18 months demonstrating a complete remodeling of the track without any residual flow. She developed a large subarachnoid hemorrhage and tentorial herniation and succumbed 5 days after the check angiogram.
Remodeling
A conventional catheter check angiogram was done in four consenting patients at a mean interval of 18 months after the device closure. There was complete remodeling with total disappearance of the track in three patients who had occluder placement in the narrow proximal segment. One patient who underwent antegrade closure of the tunnel as an infant through an arterial diagnostic catheter with embolization coils placed in the mid-segment showed partial remodeling. There was the persistence of the track without any residual flows. Computed tomographic angiography after 10 years in the first patient done in 2009 with a duct occluder placement in the atrial exit orifice leaving behind a long cul-de-sac showed partial obliteration of the track without any thrombus as she received Coumadin for atrial fibrillation.
DISCUSSION
ARAT is a congenital extracardiac communication between the aortic root at the level of the sinus of Valsalva and the right atrium.[1,3] It is differentiated from a coronary cameral fistula by its lack of side branches as well as histological resemblance to aortic tissues rather than a medium-sized artery.[14] The deficiency of the elastic lamina in the media of the aortic root causes it to bulge, form a track and rupture into the adjacent developing atria. This is proposed as an embryological explanation for these tunnels.[14,15] They are classified into anterior and posterior types depending on their origin from the aorta and course around the aortic root.[3] They take a U-turn described as the “Santa Claus hat” or “trumpet bell configuration” and drain into the superior cavoatrial junction or roof of the right atrium through one or two orifices.[9] The coronary artery usually arises adjacent to the origin of the tunnel or from the proximal part of the tunnel before the U-turn.[9] Aneurysmal enlargement of the sinoatrial nodal artery and communication to the right atrium is yet another embryological explanation.[16]
A recent attempt to summarize and categorize all congenital aorto-cardiac connections analyzed all the available publications from the first description of this tunnel in 1980 over 41 years up to 2021.[9] A total of 59 cases of ARAT were identified in the literature and reported from different institutions across the globe.[9] Apart from two publications, all others reported only 1–2 cases. The largest two publications included nine patients by Gajjar et al. and four patients by Rosenberg et al.[1,3] Our present report of 21 patients over 15 years would possibly be the largest reference for this rare anomaly. The anatomy of all the tunnels in our cohort satisfied all the criteria suggested in the differential diagnosis toolbox such as lack of side branches, normal size of the ipsilateral coronary artery, and characteristic U-turn near the aortic end to differentiate them from coronary artery fistula.[9]
We identified three different clinical presentations of these tunnels. Six out of 21 patients (29%) had a very early clinical presentation at a young age with heart failure. Two patients (10%) presented at advanced age beyond 60 years with a very dilated heart and atria, atrial fibrillation, and angina despite the absence of coronary atherosclerotic narrowing. The remaining majority of patients (61%) presented with an asymptomatic detection of a continuous murmur on routine clinical examination.
Clinical features of large aortic runoff, heart failure, and respiratory distress in a neonatal age or early infancy always warranted closure due to their life-threatening symptoms. Eleven out of 59 (19%) patients reported in an extensive review had a similar early symptomatic presentation at a young age.[9] These patients weighed under 10 kg. Attempt to close surgically without cardiopulmonary bypass by placing external clips did not succeed in one of our patients. We electively planned a transcatheter intervention in all other young patients including an 18-day-old neonate. Antenatal diagnosis of this obligatory shunt was made in one patient. Once a patient became symptomatic, heart failure arising out of this high-pressure shunt would seldom be amenable for medical management.
Six out of 59 patients published in a review article had survival beyond the fifth decade and presented with angina.[9] We also had 10% of our patients identified at an advanced age with a clinical presentation of angina in our cohort of 21 patients. While all patients quoted in the review had a surgical closure of the tunnel despite an advanced age, we resorted to transcatheter closure in both of these patients. Atrial fibrillation identified in both patients was possibly attributed to thin calcification of this tunnel running with the atrium. There was no mention of the cardiac rhythm of the six patients published in the review.[9]
ARAT closure in young asymptomatic patients was advocated to prevent ventricular volume overload, aneurysm formation of the tunnel, infective endocarditis, spontaneous rupture, thrombosis, coronary steal, calcification of the track, and aortic regurgitation from distortion of the root.[3,6,7,8] While young patients in our cohort were asymptomatic, two older patients had angina due to coronary steal despite the absence of coronary atherosclerosis. Closure of a tunnel with a dilated track would be justified for the abovementioned reasons, given the obligatory nature of the shunt.
To predict the development of complications in the future, an overflow-induced dilatation index was proposed.[9] This index factored parameters such as the diameter of the tunnel compared to the diameter of the normal coronary arteries and the age of the patient in years. The larger the tunnel, especially the distal aneurysmal segment and the older the patient, the higher rate of complication was predicted.[9] As the tunnel was very large measuring between 10 and 22 mm in all our patients, especially near the atrial end, closure was indicated.
A variety of devices including different duct occluders and vascular plugs as well as embolization coils had been used for transcatheter closure of these tunnels.
Surgical techniques included closure of the aortic and atrial ends of the tunnel with patches on cardiopulmonary bypass as well as external clipping or double ligation of the track on a beating heart. In the largest cohort of nine patients reported by Gajjar et al., there was one perioperative death from myocardial ischemia.[3] In the past 15 years, barring one patient, no others were referred for surgery from our institutions.
CONCLUSIONS
ARAT is a rare congenital anomaly with an obligatory left-to-right shunt. Transcatheter closure of this tunnel was shown to be safe and effective in this largest cohort of 21 patients, which included neonates and small infants. There was a bimodal clinical presentation with two peaks that included symptoms of heart failure in infancy and features of coronary steal with arrhythmia in the elderly. A majority of patients remained asymptomatic despite having a large, often aneurysmal tunnel. Closure of the proximal aortic end of the tunnel resulted in complete remodeling permitting early withdrawal of thromboprophylaxis. Thromboprophylaxis changed from dual antiplatelets in the early period to Coumadin recently followed by catheter closure of the coronary cameral fistula, even though the correct treatment plan remained unknown in these tunnels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rosenberg H, Williams WG, Trusler GA, Smallhorn J, Rowe RD, Moes CA, et al. Congenital aortico-right atrial communications. The dilemma of differentiation from coronary-cameral fistula. J Thorac Cardiovasc Surg. 1986;91:841–7. [PubMed] [Google Scholar]
- 2.Coto EO, Caffarena JM, Such M, Marques JL. Aorta – Right atrial communication. Report of an unusual case. J Thorac Cardiovasc Surg. 1980;80:941–4. [PubMed] [Google Scholar]
- 3.Gajjar T, Voleti C, Matta R, Iyer R, Dash PK, Desai N. Aorta-right atrial tunnel: Clinical presentation, diagnostic criteria, and surgical options. J Thorac Cardiovasc Surg. 2005;130:1287–92. doi: 10.1016/j.jtcvs.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Chidambarathanu S, Raja V, Suresh I, Sivakumar K. Aorta to right atrial tunnel with postnatal transcatheter treatment. J Fetal Med. 2016;3:175–7. [Google Scholar]
- 5.Mahesh K, Francis E, Kumar RK. Aorta to right atrial tunnel prenatal: Diagnosis and transcatheter management in a neonate. JACC Cardiovasc Interv. 2008;1:716–7. doi: 10.1016/j.jcin.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Onorato EM, Costante AM, Andreini D, Bartorelli AL. Infective endocarditis of an asymptomatic congenital aorta-right atrial tunnel: A case report. Eur Heart J Case Rep. 2020;4:1–5. doi: 10.1093/ehjcr/ytaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain J, Wani A, Kulkarni A, Yelne P. Aorta-right atrial tunnel presenting with heart failure in an adult. Heart Views. 2018;19:152–5. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_74_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altekin RE, Basarici İ, Koc S, Kucuk M, Yanikoglu A, Demir İ. Aorta-right atrial tunnel leading to heart failure. J Cardiol Cases. 2011;4:e87–9. doi: 10.1016/j.jccase.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rad EM, Hijazi ZM, Pouraliakbar H, Mirzaaghayan MR, Zamani H. Congenital aorto-cardiac connections (CACC) revisited: Introduction of a novel anatomic-therapeutic classification. Pediatr Cardiol. 2021;42:1459–77. doi: 10.1007/s00246-021-02671-5. [DOI] [PubMed] [Google Scholar]
- 10.Türkay C, Gölbaşi I, Belgi A, Tepe S, Bayezid O. Aorta-right atrial tunnel. J Thorac Cardiovasc Surg. 2003;125:1058–60. doi: 10.1067/mtc.2003.87. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal SK, Sai V, Iyer VR. Imaging features of aorto-right atrial tunnel: A report of two cases. Congenit Heart Dis. 2007;2:429–32. doi: 10.1111/j.1747-0803.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 12.Sivakumar K, Shahani JM, Francis E. Transcatheter closure of aortico right atrial tunnel – A rare cardiac anomaly. Congenit Heart Dis. 2006;1:324–6. doi: 10.1111/j.1747-0803.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- 13.Baykan A, Narin N, Ozyurt A, Uzum K. Aorta-right atrial tunnel closure using the transcatheter technique: A case of a 3-year-old child. Cardiol Young. 2013;23:457–9. doi: 10.1017/S1047951112001151. [DOI] [PubMed] [Google Scholar]
- 14.Jainandunsing JS, Linnemann R, Maessen J, Natour NE, Lorusso R, Gelsomino S, et al. Aorto-atrial fistula formation and therapy. J Thorac Dis. 2019;11:1016–21. doi: 10.21037/jtd.2019.02.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KN, Cho KI, Kim JJ, Kang JH, Goo JJ, Lee JY, et al. A case of aorta-right atrial tunnel presented with an asymptomatic murmur. Korean Circ J. 2013;43:640–3. doi: 10.4070/kcj.2013.43.9.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyisoy A, Celik T, Celik M, Sag C. Aorta-right atrial tunnel: An interesting type of a congenital coronary artery anomaly. Korean Circ J. 2014;44:193–5. doi: 10.4070/kcj.2014.44.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]