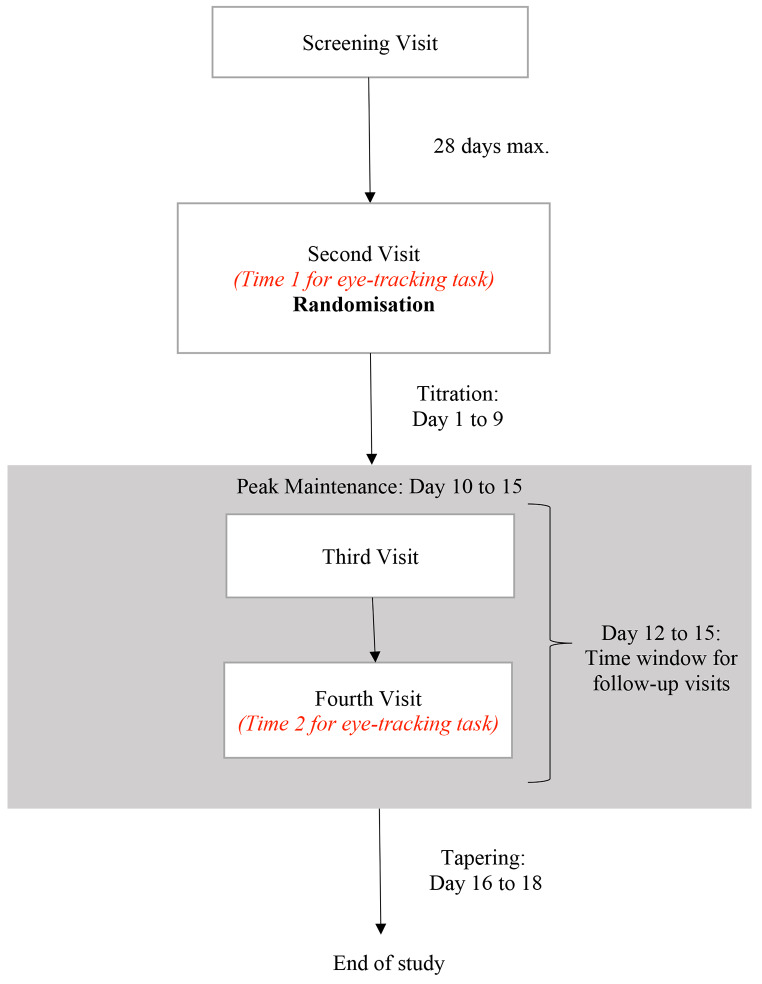
Fig. 2.
Study Timeline. Participants attended the screening visit and completed eligibility assessment. If eligible, they were invited to attend the second visit no more than 28 days after the screening visit. Participant completed the eye-tracking task for the first time during the second visit. After completion the baseline measurement, they were randomized to receive either pramipexole or placebo and took their first dose of the assigned medication at the visit. Pramipexole was initiated at a dose of 0.25 mg/day of pramipexole salt and was increased by 0.25 mg every three days to a maximum of 1 mg/day (achieved on day 10). Participants continue to maintain 1 mg dose between day 10 and 15. The third and fourth follow-up visits are completed within the day 12 to 15 time window. Participants were then tapered off the medication by reducing the dose by 0.25 mg per day over 3 days between day 16 to 18