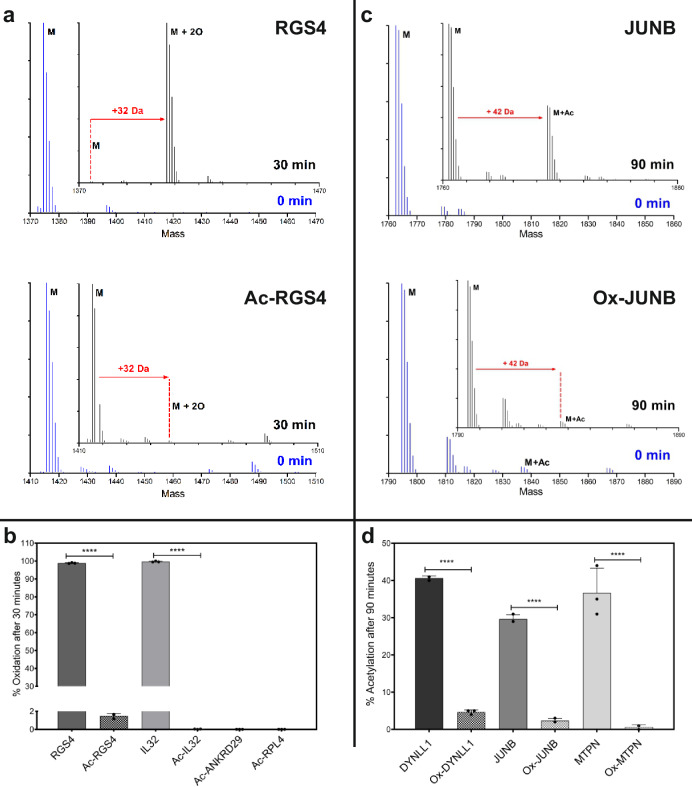
Fig. 7. Nt-acetylation abrogates oxidation by ADO in vitro and vice versa.
a Deconvoluted MS spectra showing oxidation (32 Da increase) of RGS42-15 (RGS4) and N-terminally acetylated RGS42-15 (Ac-RGS4) before (blue, 0 min) and after (black, 30 min) incubation with HsADO at 37 °C (100 µM peptide, 0.1 µM ADO). b Oxidation of other acetylated ADO substrate peptides (Fig. 2a) compared to oxidation of RGS42-15 and IL322-15 peptides after 30 min (100 µM peptide, 0.1 µM HsADO, 37 °C). c Deconvoluted MS spectra showing acetylation (42 Da increase) of JUNB2-15 (JUNB) and N-terminally oxidised JUNB2-15 (Ox-JUNB) before (blue, 0 min) and after (black, 30 min) incubation with NatA at 37 °C (100 µM peptide, 0.15 µM NatA). d Acetylation of other oxidised NatA substrates peptides (Fig. 6a) compared to acetylation of non-oxidised NatA substrates peptides after 90 min (100 µM peptide, 0.15 µM NatA, 37 °C). In (b, d), data are presented as mean ± standard deviation, n = 3. Statistical significance determined using 2-way ANOVA with Bonferroni’s multiple comparison post-test: ****p ≤ 0.0001. Source data are provided as a Source Data file.