Figure 1: Trial profile.
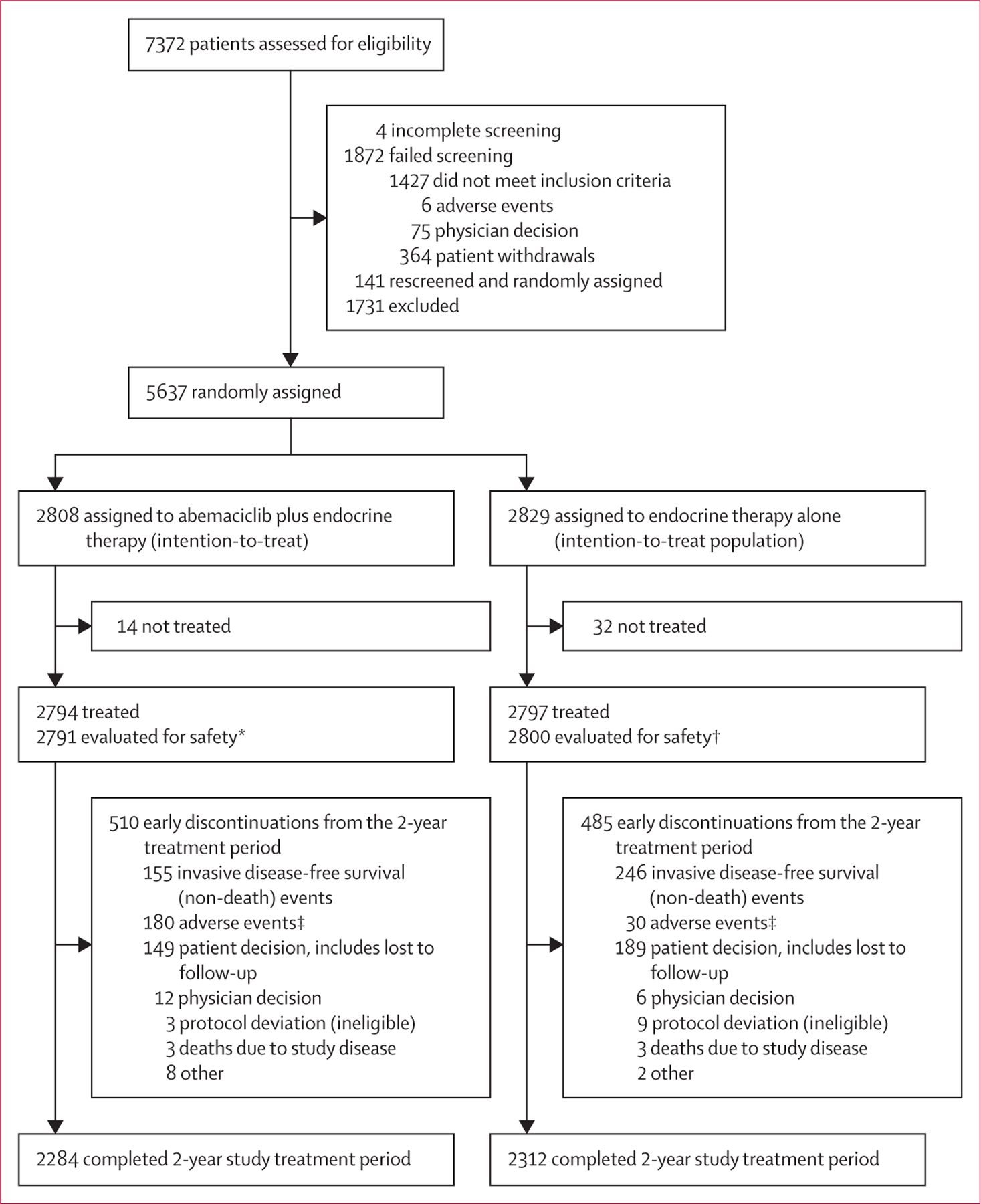
*Four patients randomly assigned to the abemaciclib group only received endocrine therapy and were evaluated for safety in the control group. †One patient randomly assigned to the control group received abemaciclib and was evaluated for safety in the abemaciclib group. ‡13 fatal adverse events in the abemaciclib plus endocrine therapy group and eight fatal adverse events in the endocrine therapy alone group.
