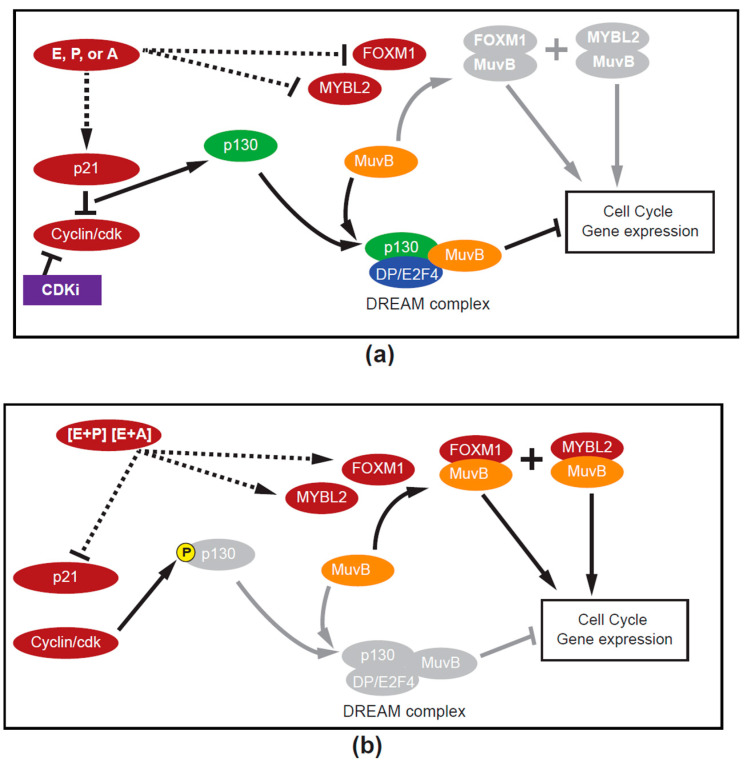
Figure 6.
(a) DREAM “ON”. A hypothetical model proposing that single-hormone stimulation, either estrogen (E), progesterone (P) or androgens (A), is associated with increases in p21. The increase in p21 and its inhibition of cyclin-dependent kinase activity will lead to hypophosphorylation of the retinoblastoma family proteins, allowing them to preferentially compete for the LIN52 member of MuvB core proteins. The resulting stable DREAM repressor complex binds to the CHR element of cell cycle genes through another MuvB core protein (LIN54) and E2F binding sites via E2F4/5-DP to suppress cell cycle genes, causing reversible cell cycle arrest (quiescence) and suppression of the MuvB target genes FOXM1 and MYBL2/B-MYB. Formation of the DREAM complex would be facilitated by cyclin-dependent kinase inhibitor (CDKi) therapy. (b) DREAM “OFF”. A hypothetical model proposing that dual hormone stimulation (e.g., estrogen plus progesterone or estrogen plus an androgen) could (dashed line) inhibit p21 (see Figure 3). The inhibition of p21 would allow cyclin-dependent kinases to phosphorylate the retinoblastoma-like protein p130 releasing the E2F transcription factor, which would increase the pro-proliferative MuvB co-factor MYBL2. This would competitively bind the MuvB core proteins, removing them from the dimerization partner (DP/E2F4, p130) complex and activating the same cell cycle genes and increasing both FOXM1 and MYBL2/B-MYB, resulting in proliferation. Both figures reprinted and modified from [25] (p. 6, Figure 5C). Creative Commons License. Modified by shading and substitution of hormone combinations instead of estrogen as the initiating stimulus for influencing cell cycle gene expression.