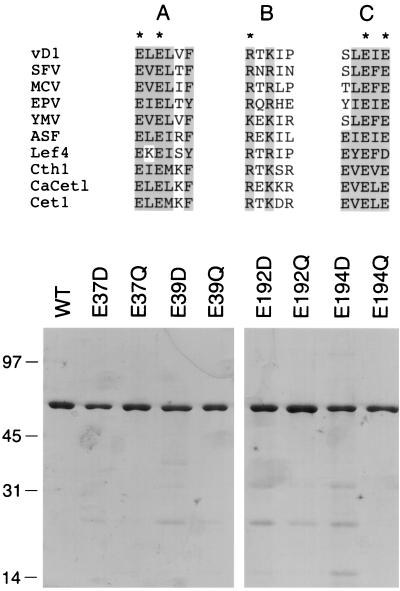
FIG. 3.
Purification of wild-type and mutated versions of vaccinia virus D1(1-545)p. Conserved motifs A, B, and C of the RNA triphosphatases of vaccinia virus (vD1), Shope fibroma virus (SFV), molluscum contagiosum virus (MCV), Melanoplus sanguinipes entomopoxvirus (EPV), Yaba monkey tumor virus (YMV), African swine fever virus (ASF), Autographa californica baculovirus (Lef4), S. cerevisiae (Cth1 and Cet1), and C. albicans (CaCet1) are aligned in the top panel. Vaccinia virus D1 residues that are conserved in the other proteins are shaded. The five amino acids in vD1(1-545)p that were found by alanine scanning to be essential for triphosphatase activity are indicated by asterisks. Protein purification is shown in the bottom panel. Aliquots (0.5 μg) of the phosphocellulose preparations of recombinant wild-type (WT) vD1(1-545) and the indicated mutant proteins were electrophoresed through a 10% polyacrylamide gel containing 0.1% SDS. Polypeptides were visualized by staining with Coomassie Blue dye. The positions and sizes (in kilodaltons) of marker proteins are indicated on the left.