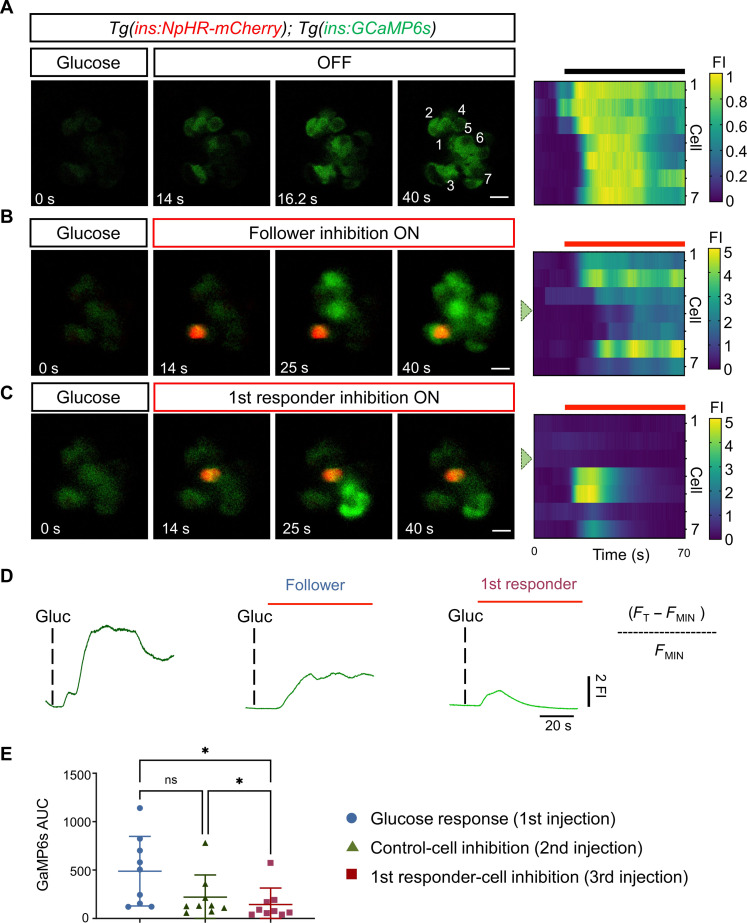
Fig. 2. Optogenetic inhibition of first-responder cells using NpHR3.0 decreases the pan-islet glucose-stimulated calcium influx.
(A) Images and raster plots from a time-lapse recording (6 Hz) of the islet after a glucose stimulation in Tg(ins:GCaMP6s); Tg(ins:eNpHR3.0-mCherry) double transgenic larvae. (B and C) Images from the islet shown in (A) after a glucose injection and simultaneous in vivo optogenetic inhibition with a green laser (λ = 561). The optogenetic inhibition was performed with the region of interest (ROI) scan encompassing the area of one follower or a first-responder β cell. Glucose was injected at 5-min intervals. The red signal corresponds to NpHR-mCherry fluorescence. (D) The traces show the normalized GCaMP6 fluorescence traces and the peak in calcium influx after glucose stimulation and upon the light-mediated inhibition of a control or a first-responder β cell. FI, fluorescence intensity. The red bars indicate the period of green laser exposure. (E) Quantification of the area under the curve (AUC) reflecting 250 frames (37.5 s) of normalized GCaMP6 fluorescence after glucose injection, follower-cell inhibition, and first-responder cell inhibition (n = 9 independent samples) [one-way paired analysis of variance (ANOVA), Tukey’s correction. *P = 0.0226 “glucose response versus first-responder cell” and *P = 0.0259 “control cell versus first-responder cell”; ns, not significant). Data are shown as means ± SD. Scale bars, 10 μm.