The purpose of this report is twofold—to report the potential for magnetic resonance angiography to miss sizeable intracranial aneurysms and to highlight the value of simple, quantitative clinical reasoning when interpreting the results of diagnostic tests.
Subarachnoid haemorrhage accounts for a quarter of all cerebrovascular deaths, and over a third of those who survive have major neurological deficits.1,2 Intracranial aneurysms, the commonest cause of subarachnoid haemorrhage, may present with rupture, mass effect, or, rarely, with emboli phenomena in large aneurysms. The typical presentation of rupture is headache of instantaneous onset that remains continuous and is often associated with nausea, vomiting, meningism, or loss of consciousness. About a third of patients with aneurysmal subarachnoid haemorrhage will re-bleed, and this is a major cause of poor outcome.3,4 The risk of re-bleeding peaks on the first day and then declines.5 Most studies therefore support the need for surgery soon after rupture, and delay in diagnosis or misdiagnosis as migraine or meningitis can have catastrophic consequences.6,7
Unruptured intracranial aneurysms causing mass effect may present as pain or neurological deficit depending on the site and size of the aneurysm. Such aneurysms are often large or giant,8 and, as most intracranial aneurysms occur at the junction of the internal carotid and posterior communicating artery, the commonest clinical sign is oculomotor palsy. Unruptured intracranial aneurysms causing mass effect are at high risk of subsequent rupture, estimated at 6% a year.9
The optimal method for detecting intracranial aneurysms is intra-arterial digital subtraction angiography. This procedure carries an associated morbidity of transient or permanent neurological disability (of 1% and 0.5% respectively).10 This associated morbidity and increasing access to magnetic resonance imaging has led to interest in the use of magnetic resonance angiography for assessing patients at high risk of symptomatic intracranial aneurysm.
Case reports
Case 1
A 21 year old man developed a headache of instantaneous onset. The headache improved overnight, becoming persistent and centred behind the left eye. Ten days later he noticed drooping of his left eyelid and double vision. Clinical examination revealed a left, complete, oculomotor palsy. Results of magnetic resonance imaging of the head and intracranial magnetic resonance angiography (fig 1) were reported to be normal by a senior consultant neuroradiologist who was aware of the clinical suspicion of a posterior communicating artery aneurysm. The patient's physician reasoned that, since magnetic resonance angiography has a sensitivity to detect intracranial aneurysms of ⩾95%, the probability of a posterior communicating artery aneurysm was ⩽5%.
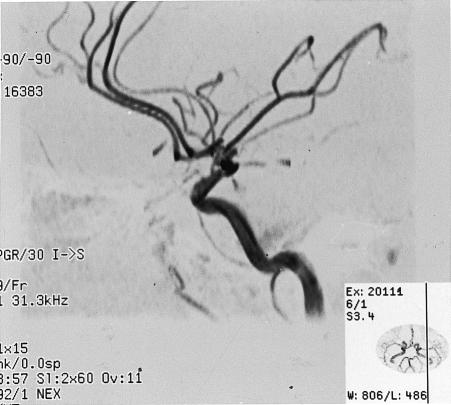
Figure 1.
Case 1: magnetic resonance angiography with maximum intensity projection has normal appearance (top), whereas intra-arterial digital subtraction angiography (injection of left internal carotid artery) shows a large left posterior communicating artery aneurysm (bottom)
The diagnosis of posterior communicating artery aneurysm was discounted, and an alternative cause for the patient's oculomotor palsy was sought. Subsequent failure to identify another cause led to a re-evaluation, and cerebral intra-arterial digital subtraction angiography revealed a left 8 mm posterior communicating artery aneurysm (fig 1). The aneurysm was surgically clipped without complication.
Case 2
A 48 year old woman developed a headache of instantaneous onset. The headache improved over the course of a few hours, becoming centred behind the right eye. Two days later she became drowsy, photophobic, and meningitic. Computed tomography of the head gave normal results. Examination of the cerebrospinal fluid revealed bloodstained fluid (opening pressure not recorded), a red blood cell count of 180×109/l, white blood cell count 210×106/l (70% lymphocytes, 30% neutrophils), and glucose concentration 2.2 mmol/l (serum concentration 8.0 mmol/l). A diagnosis of “bloody tap” and meningitis was made, and she received benzylpenicillin.
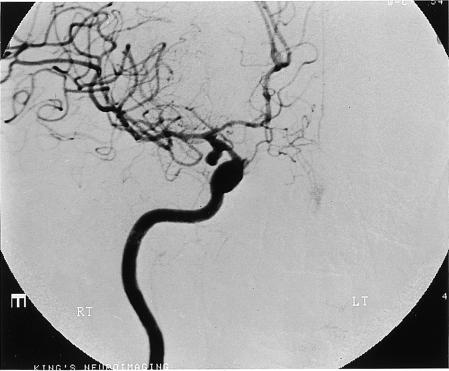
The next day she deteriorated, and partial oculomotor palsy of the right pupil was noted. The results of magnetic resonance imaging of the head (before and after use of gadolinium contrast agent) and magnetic resonance angiography were reported by a senior consultant neuroradiologist, who was aware of the clinical suspicion of a posterior communicating artery aneurysm, as showing meningeal enhancement consistent with subarachnoid haemorrhage but with no sign of an aneurysm (fig 2). In view of the strong clinical suspicion of intracranial aneurysm, cerebral intra-arterial digital subtraction angiography was performed and revealed a right 8 mm posterior communicating artery aneurysm (fig 2). The aneurysm was surgically clipped without complication.
Figure 2.
Case 2: magnetic resonance angiography with maximum intensity projection has normal appearance (top), whereas intra-arterial digital subtraction angiography (injection of right internal carotid artery) shows a large right posterior communicating artery aneurysm (bottom)
Comment
These cases highlight the potential for high quality magnetic resonance angiography to miss sizeable intracranial aneurysms and thereby contribute to delayed or wrong diagnosis. In both cases the images were reported by a senior consultant neuroradiologist aware of the clinical suspicion of intracranial aneurysm and after analysis of maximum intensity projections and axial base images.
The major clinical lesson, however, concerns the degree of reliance placed on a negative test result when clinical features strongly suggested otherwise. In case 1 the patient's initial physician reasoned that, because magnetic resonance angiography has a ⩾95% sensitivity to detect intracranial aneurysms, the probability of the patient having an aneurysm was ⩽5%. This reasoning fails to consider the probability of intracranial aneurysm based on both the clinical features and the test result. The probability of a diagnosis based on both probabilities is termed the posterior (post-test) probability and is calculated using Bayes's rules.11,12
Of key importance in bayesian calculations is the accurate estimation of prior clinical probability and test sensitivity and specificity. For case 1, we estimate the clinical probability of posterior communicating artery aneurysm to be high (90%). This is based on the fact that the patient's oculomotor palsy involved pupillary fibres (characteristic of a “surgical third”) and that isolated painful third nerve palsies are the hallmark of posterior communicating artery aneurysms. The test sensitivity (the ability of magnetic resonance angiography to detect intracranial aneurysms) is ⩾95% for aneurysms >6 mm in diameter or when axial base and spin-echo images are reviewed as well.1 The specificity of magnetic resonance angiography (the probability of a negative result when disease is absent) ranges from 92% to 100%.13,14 Assuming a prior clinical probability of an aneurysm of 90% and magnetic resonance angiography sensitivity and specificity of 95% and 92% respectively, the probability of a posterior communicating artery aneurysm after a negative magnetic resonance angiography is reduced from 90% to 32.85% (fig 3). Thus, in these circumstances a negative result from magnetic resonance angiography cannot be used to exclude the diagnosis. The delay in diagnosis occurred because insufficient weight was given to the clinical findings and too much weight to the magnetic resonance angiography result. For case 2, however, once the misdiagnosis of meningitis was discarded, appropriate weight was given to the clinical probability of aneurysm, and intra-arterial digital subtraction angiography was undertaken immediately despite the negative result from magnetic resonance angiography.
Figure 3.
Probability of a posterior communicating artery aneurysm given a negative or positive result from magnetic resonance angiography and a prior clinical probability of 90%. Sensitivity and specificity of angiography are 95% and 92% respectively. Probabilities are expressed between 0.0 (0%) and 1.0 (100%)
We have avoided using the terms positive and negative predictive values. The positive predictive value is the probability a disease is present given a positive test result. The negative predictive value is the probability a disease is absent given a negative result. The probability that a disease is present given a negative result is therefore 1−negative predictive value. The term posterior probability avoids this potential confusion and simply refers to how likely a patient is to have a disease given the result of a diagnostic test. We could have calculated posterior odds rather than posterior probability, but we have avoided the use of odds and likelihood ratios because they require the conversion of probabilities to odds. As clinical likelihood, sensitivity, and specificity are all usually expressed as probabilities rather than odds, we prefer the more intuitive posterior probability to express the post-test likelihood of a disease.
Fig 4 shows how the probability of a disease after a diagnostic test is critically dependent on the prior clinical probability. For example, a small reduction in the prior clinical probability of an intracranial aneurysm from 90% to 50% reduces the probability of aneurysm given a negative result from magnetic resonance angiography from 33% to 5%. Such a reduction may not be enough to rule out the diagnosis with certainty, but it might be enough to question it. The reliance of posterior probability on prior clinical suspicion is daunting since even small errors in the estimation of clinical suspicion can substantially affect the final decision about whether a disease is present. Methods to quantify clinical suspicion are described elsewhere.13,14 Doctors' diagnostic opinions can differ because some are better than others in their ability to correctly estimate the prior clinical probability of a disease, in their knowledge of test sensitivity and specificity, or in their intuitive ability with Bayesian statistics.
Figure 4.
Influence of prior clinical probability on the probability of a disease after a negative or positive test result. Test sensitivity and specificity are 95% and 92% respectively
The formulas in fig 3 provide a simple and convenient method for calculating the probability of a disease based on both the prior clinical probability and the result of a diagnostic test.
The probability of a disease following a diagnostic test is critically reliant on prior clinical probability
Footnotes
Funding: No additional funding.
Competing interests: None declared.
References
- 1.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123:205–221. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid haemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 4.Roos YBWEM, Beenen LFM, Groen RJM, Albrecht KW, Vermeulen M. Timing of surgery in patients with aneurysmal subarachnoid haemorrhage: rebleeding is still the major cause of poor outcome in neurosurgical units that aim early surgery. J Neurol Neurosurg Psychiatry. 1997;63:490–493. doi: 10.1136/jnnp.63.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Co-operative study on the timing of aneurysm surgery. Part 2: Surgical results. J Neurosurg. 1990;73:37–47. doi: 10.3171/jns.1990.73.1.0037. [DOI] [PubMed] [Google Scholar]
- 6.Mayer PL, Awad IA, Todor R, Harbaugh K, Varnavas G, Lansen TA, et al. Misdiagnosis of symptomatic cerebral aneurysm: prevalence and correlation with outcome at four institutions. Stroke. 1996;27:1558–1563. doi: 10.1161/01.str.27.9.1558. [DOI] [PubMed] [Google Scholar]
- 7.Neil-Dwyer G, Lang D. ‘Brain attack’—aneurysmal subarachnoid haemorrhage: death due to delayed diagnosis. J R Coll Physicians Lond. 1997;31:49–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Raps EC, Rogers JD, Galetta SL, Solomon RA, Lennihan L, Klebanoff LM, et al. The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol. 1993;50:265–268. doi: 10.1001/archneur.1993.00540030031010. [DOI] [PubMed] [Google Scholar]
- 9.Wiebers DO, Whisnant JP, Sundt TM, Jr, O'Fallon WM. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23–29. doi: 10.3171/jns.1987.66.1.0023. [DOI] [PubMed] [Google Scholar]
- 10.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid haemorrhage, cerebral aneurysm and arteriovenous malformation: a meta-analysis. Stroke. 1999;30:317–320. doi: 10.1161/01.str.30.2.317. [DOI] [PubMed] [Google Scholar]
- 11.Sackett DL, Haynes RB, Tugwell P. The interpretation of diagnostic data. In: Sackett DL, Haynes RB, Tugwell P, editors. Clinical epidemiology. Boston: Little, Brown; 1985. pp. 59–138. [Google Scholar]
- 12.Gross R. Making medical decisions. Philadelphia: American College of Physicians; 1999. [Google Scholar]
- 13.Horikoshi K, Fukamachi A, Nishi H, Fukasawa I. Detection of intracranial aneurysms by three-dimensional time-of-flight magnetic resonance angiography. Neuroradiology. 1994;36:203–207. doi: 10.1007/BF00588131. [DOI] [PubMed] [Google Scholar]
- 14.Wilcock D, Jaspan T, Holland I, Cherryman G, Worthington B. Comparison of magnetic resonance angiography with conventional angiography in the detection of intracranial aneurysms in patients presenting with subarachnoid haemorrhage. Clin Radiol. 1996;51:330–334. doi: 10.1016/s0009-9260(96)80109-7. [DOI] [PubMed] [Google Scholar]