Fig. 1. Termini restraining of small membrane proteins facilitates their entire structure determination process.
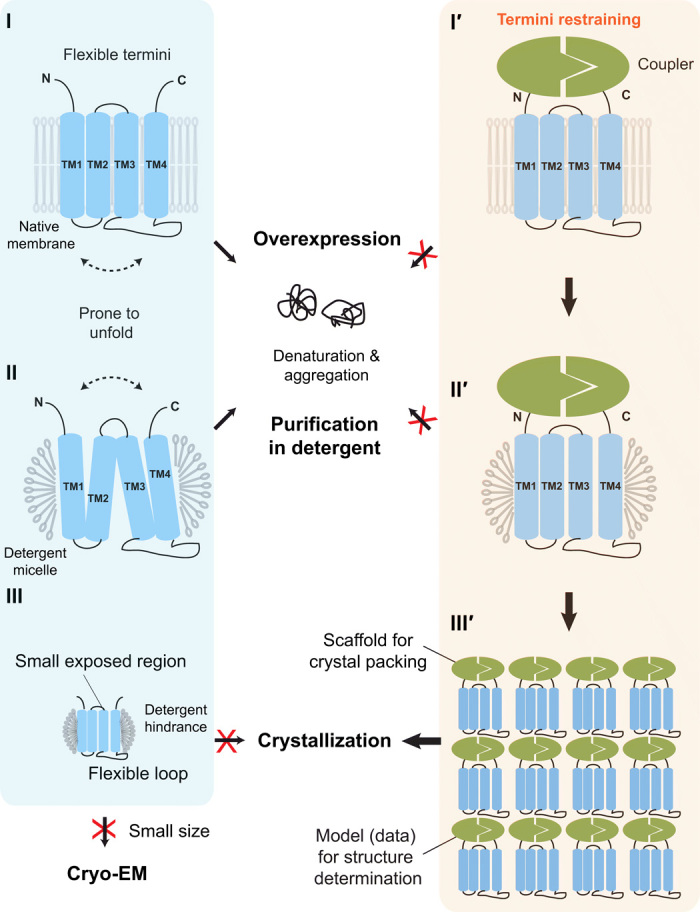
(Left) Difficulties with traditional structural biology approaches (blue box). (I) Overexpression of membrane proteins increases their tendency to unfold and aggregate in cells. The unfolding involves large relative motions between TMs (dashed arrow). (II) Protein purification in detergents is often disruptive to the native folded state of membrane proteins. (III) Small membrane proteins in detergent micelles often contain either small exposed regions or large flexible loops, both of which increase the difficulty of making crystal contact. These proteins are also too small for structural determination by cryo-EM. (Right) Termini-restraining strategy (orange box). (I′) Two associable protein entities (coupler; green) are fused to the flexible N and C termini of a membrane protein (blue), providing a loose restraint to stabilize its folded state during protein overexpression in cells (I′) and protein purification in detergents (II′). (III′) Introducing the coupler protein also provides a large surface for crystal packing and facilitates structure determination.
