Fig. 3. Deletion of Dcaf1 selectively eliminates proliferating cells in vitro.
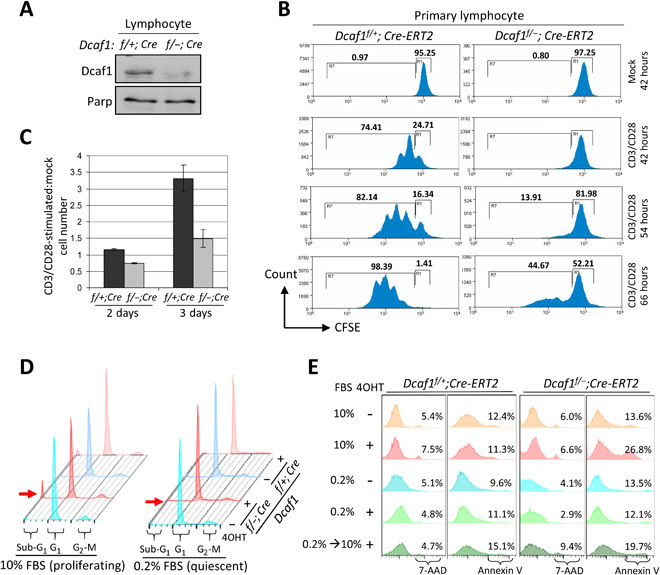
(A) Immunoblotting of primary lymphocyte lysates derived from Dcaf1f/+;Cre-ERT2 and Dcaf1f/−;Cre-ERT2 mice 6 days after tamoxifen injection. (B) Primary lymphocytes were labeled with CFSE and followed by CD3/CD28 stimulation or mock. Cells were cultured in vitro for 42, 54, or 66 hours before collection. Flow cytometric analysis for CFSE was used to determine the proliferation, gated by the 7-AAD–negative T cells. (C) Primary T cells were costimulated with CD3/CD28 or mock-treated and then plated at equal cell numbers. The total cell numbers following activation, expressed relative to the numbers of mock-treated cells, are reported at 2 and 3 days after stimulation. (D) MEFs were cultured in 10 or 0.2% FBS 1 day before 4OHT treatment for 3 days. DNA content was analyzed by PI staining followed by flow cytometry. Red arrow indicates sub-G1 population, representing apoptotic cells. (E) MEFs were cultured in 10 or 0.2% FBS 1 day before 4OHT treatment for 4 days. One group of MEFs was restimulated by 10% FBS after 2-day serum starvation. Cell death and apoptosis analysis was carried out by staining with 7-AAD and annexin V, respectively.
