Fig. 4. Ribosome assembly factor PWP1 is a substrate of CRL4DCAF1 E3 ubiquitin ligase.
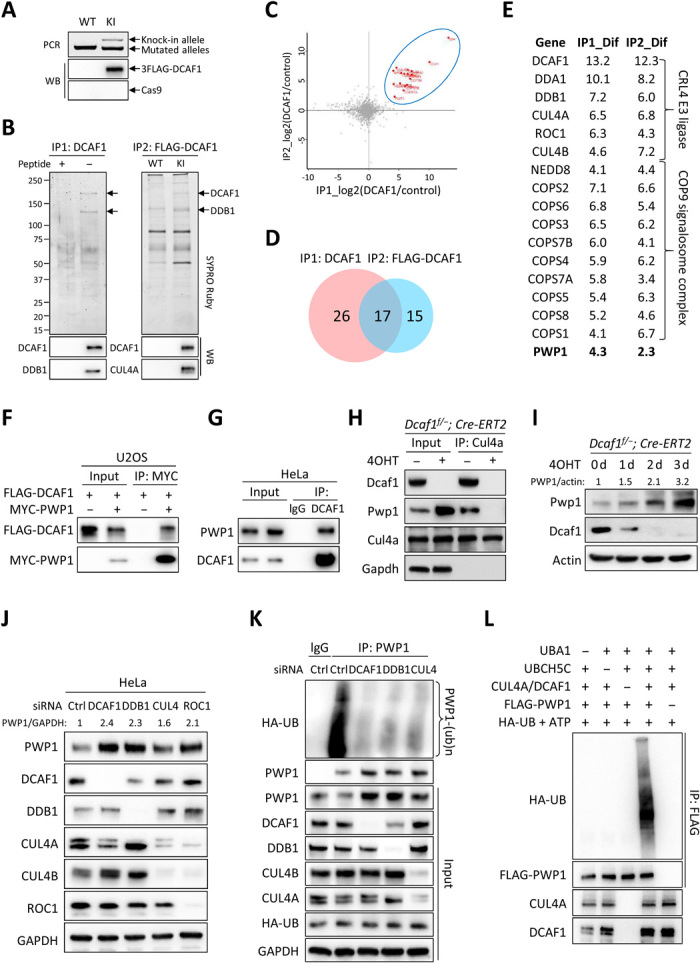
(A) 3xFLAG knock-in (KI) to endogenous DCAF1 in HeLa cells verified by genomic PCR and immunoblotting. WT, wild type; WB, Western blot. (B) Immunopurification of two endogenous DCAF1 complexes from HeLa cells. Molar excess competing antigen peptides added in control immunocomplexes. (C) Scatter plot of log2 ratios of 626 proteins identified by mass spectrometry analyses of two DCAF1 immunocomplexes. (D) Venn diagram of proteins with greater than fourfold abundance change. (E) List of 17 DCAF1-interacting proteins identified in both DCAF1 immunocomplexes. (F) Interaction between ectopically expressed PWP1 and DCAF1 in U2OS cells by coimmunoprecipitation (co-IP). (G) Endogenous interaction between DCAF1 and PWP1 in HeLa cells treated with 20 μM MG132 for 6 hours. (H) Dcaf1f/−;Cre-ERT2 MEFs with 200 nM 4OHT or dimethyl sulfoxide (DMSO) for 2 days. Protein-protein interactions by co-IP. (I) MEFs with 200 nM 4OHT for indicated days. (J) HeLa cells transfected with small interfering RNAs (siRNAs) targeting indicated genes individually. (K) HeLa cells stably expressing HA-tagged ubiquitin transfected with siRNAs targeting indicated genes and treated with MG132. Endogenous PWP1 was immunoprecipitated and immunoblotted. (L) Immunopurified PWP1 protein incubated with CRL4DCAF1 E3 immune complex in the presence or absence of UBA1 (E1), UBCH5C (E2), adenosine triphosphate (ATP), and ubiquitin in vitro for 1 hour. Ubiquitylated PWP1 was immunoprecipitated and immunoblotted.
