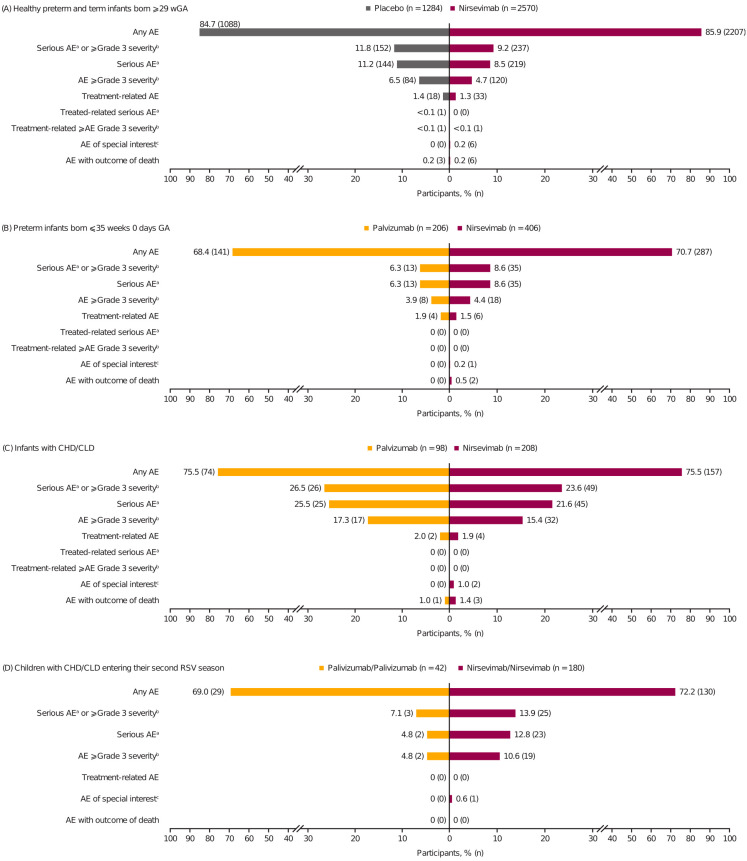
Figure 1.
Overall summary of AEs through 360 days post-dose in (A) healthy term and preterm infants ≥ 29 wGA (includes infants from Phase 2b weighing < 5 kg and the full MELODY enrollment cohort) and palivizumab-eligible infants at higher risk of severe RSV disease from the MEDLEY trial, including (B) preterm infants ≤ 35 weeks 0 days GA without CLD or CHD, (C) infants with CHD/CLD (all entering their first RSV season), and (D) children with CHD/CLD entering their second RSV season (before the second season, children with CHD/CLD randomized to nirsevimab in the first season received a single IM dose of 200 mg nirsevimab followed by 4× once-monthly IM doses of placebo [nirsevimab/nirsevimab], and those randomized to palivizumab in the first season were re-randomized 1:1 to either a single IM dose of 200 mg nirsevimab followed by 4× once-monthly IM doses of placebo [palivizumab/nirsevimab] or 5× once-monthly IM doses of palivizumab [15 mg/kg per dose; palivizumab/palivizumab]). Participants with multiple events in the same category were counted once in that category; participants with events in >1 category were counted once in each category. (a) Defined as death, life-threatening, requiring inpatient hospitalization, prolongation of existing hospitalization, persistent or significant disability/incapacity, important medical event, or congenital anomaly/birth defect. (b) Grade 1: mild; Grade 2: moderate; Grade 3: severe; Grade 4: life-threatening; Grade 5: fatal. (c) Included immediate type I hypersensitivity reactions (including anaphylaxis), immune complex disease, and thrombocytopenia. Abbreviations: AE, adverse event; CHD, congenital heart disease; CLD, chronic lung disease of prematurity; GA, gestational age; IM, intramuscular; RSV, respiratory syncytial virus; wGA, weeks’ gestational age.