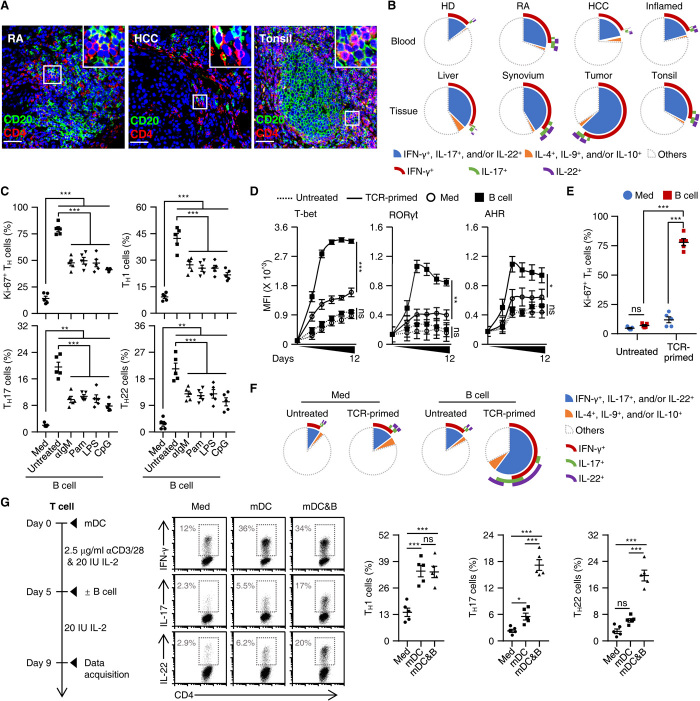
Fig. 1. Identifying helper functions of B cells in generation of inflammatory TH subsets.
(A) Confocal microscopy analysis of expression of CD4 (red, TH cells) and CD20 (green, B cells) in samples from RA synovial tissue (n = 8), HCC tissue (n = 11), and inflamed tonsils (n = 11). Scale bars, 50 μm. (B) FACS analysis of IFN-γ, IL-17, IL-22, IL-4, IL-9, and IL-10 in TH cells from paired blood and tissue samples from healthy donors (HD) and patients with RA, HCC, or inflamed tonsils (each n = 5). (C) Purified total B cells were left untreated or were stimulated with an anti-IgM antibody (αIgM), Pam3CysSK4 (Pam), LPS, or CpG-DNA ODN (CpG) for 18 hours and then cultured with autologous T cells for 9 days. Expression of Ki-67 and indicated cytokines in TH cells were detected by FACS (n = 5). (D to F) Purified T cells were cultured in medium or with autologous total B cells for 9 days (E and F) or indicated times (D) in the presence or absence of TCR triggering, as described in Materials and Methods. Expression of indicated transcription factors (D), Ki-67 (E), and inflammatory cytokines (F) in TH cells was detected by FACS (n = 5). MFI, mean fluorescence intensity. (G) Purified T cells were cultured in medium or with mature myeloid dendritic cells (mDCs) in the presence or absence of total B cells as described in workflow. FACS analysis was performed to illustrate expression of IFN-γ, IL-17, and IL-22 in TH cells (n = 5). Data are presented as means ± SEM of four independent experiments (C to E and G). ns, nonsignificant. *P < 0.05, **P < 0.01, and ***P < 0.001 [one-way ANOVA test for (C), (D), and (G); two-way ANOVA test for (E)].