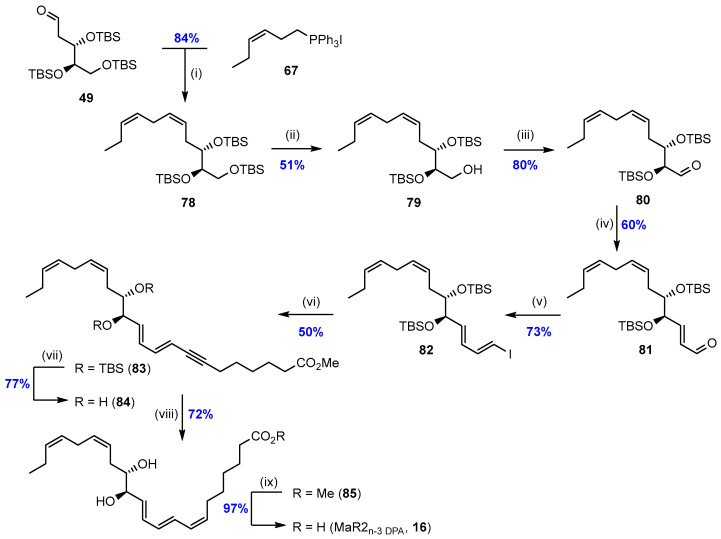
Scheme 11.
Final steps in the stereoselective synthesis of MaR2n-3 DPA (16) [59]. Reagents and conditions: (i) NaHMDS, CH2Cl2, −78 °C; (ii) para-toluene sulfonic acid, MeOH, −20 °C; (iii) DMP, NaHCO3, CH2Cl2; (iv) (triphenylphosphoranylidene)acetaldehyde, toluene, Δ; (v) CrCl2, dioxane, THF, CHI3, 0 °C; (vi) CuI, Pd(PPh3)4 (5 mol%), alkyne 76, Et2NH; (vii) TBAF, THF; (viii) Lindlar’s catalyst (Pd/CaCO3), EtOAc/pyridine/1-octene, H2 (g); (ix) LiOH, H2O, MeOH, 0 °C.