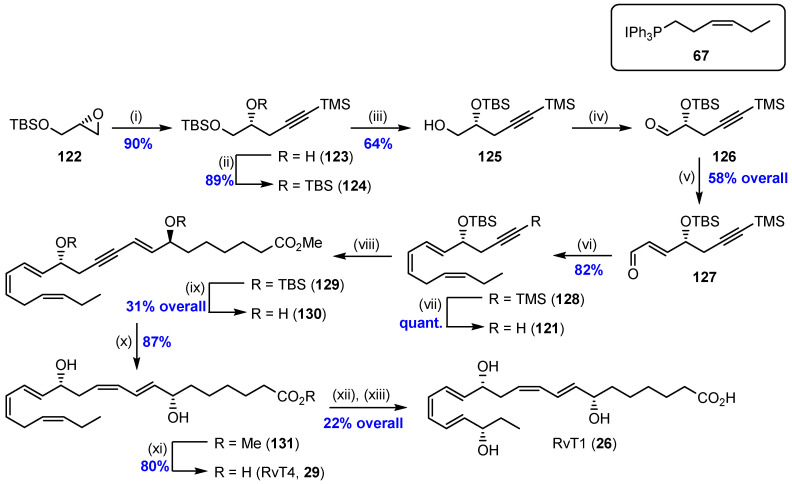
Scheme 19.
Preparation of the ω-fragment 121 and coupling of fragments to complete the synthesis of RvT4 (29). RvT1 (26) was prepared from enzymatic hydroxylation of RvT4 (29) [62]. Reagents and conditions: (i) Trimethylsilylacetylene, n-BuLi, BF3·Et2O, THF, –78 °C; (ii) TBSCl, imidazole, 4-DMAP, CH2Cl2, 0 °C to rt; (iii) CSA, CH2Cl2/MeOH 1/1, 0 °C; (iv) Dess–Martin periodinane, CH2Cl2, rt; (v) (Triphenylphosphoranylidene)acetaldehyde, MeCN, 30 °C; (vi) 67, n-BuLi, THF, –78 °C to 0 °C; (vii) K2CO3, CH3OH, rt; (viii) 105, Pd(PPh3)4, CuI, piperidine, benzene, rt; (ix) CH3COCl, MeOH, 0 °C to rt; (x) Zn(Cu/Ag), CH3OH, H2O, 40–45 °C; (xi) 1 N LiOH, MeOH/H2O 1/1, 0 °C to rt; (xii) lipoxidase type I-B from soybean, 0.01 M borate buffer pH 10.7, rt; (xiii) TCEP-HCl, rt.