Fig. 2. 3D bioprinted gradient cartilage scaffold for implantation.
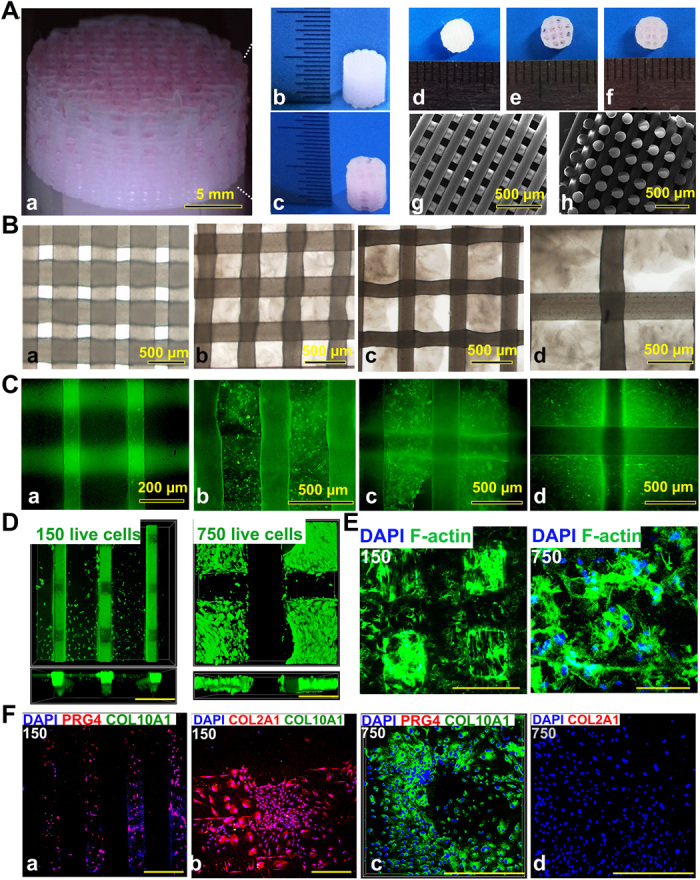
(A) Gross appearance of (a) human-scale and (b and c) rabbit-scale cartilage scaffold (b, NG with 150-μm spacing; c, NG with 750-μm spacing). Top view of the rabbit cartilage scaffold is also shown (d, NG with 150-μm spacing; e, NG with 750-μm spacing; f, gradient scaffold with 150- to 750-μm spacing) atop of the SEM images (g, horizontal section; h, vertical section) taken for the 150-μm NG scaffold to demonstrate the precise alignment of the PCL fibers in the printed scaffold. (B) Deconstruction of the gradient scaffold. The structure of the gradient scaffold was deconstructed into four layers. Microscopic appearance of the hydrogel-PCL composite structure in each layer demonstrated good interconnectivity and delicate, orderly aligned structure for each layer. (C and D) Good cell viability is shown respectively for superficial and deep layers after printing with live/dead assay (green, live cells; red, dead cells) (C) under a microscope and (D) under a confocal microscope. DAPI, 4′,6-diamidino-2-phenylindole. (E) Cell spreading in superficial and deep layers with cytoskeleton staining. (F) Immunostaining for cartilage markers in superficial and deep layers. Expression of COL2A1 and PRG4, the lubrication markers, was significantly higher in the superficial layers with small pore size (a and b), while the chondrogenic cells in the deep layers (c and d) mostly presented with hypertrophic phenotype (COL10A1 expression). Photo credit: Ye Sun, First Affiliated Hospital of Nanjing Medical University.
