Abstract
The genus Borrelia has been divided into Borreliella spp., which can cause Lyme Disease (LD), and Borrelia spp., which can cause Relapsing Fever (RF). The distribution of genus Borrelia has broadened due to factors such as climate change, alterations in land use, and enhanced human and animal mobility. Consequently, there is an increasing necessity for a One Health strategy to identify the key components in the Borrelia transmission cycle by monitoring the human-animal-environment interactions. The aim of this study is to summarize all accessible data to increase our understanding and provide a comprehensive overview of Borrelia distribution in the Mediterranean region. Databases including PubMed, Google Scholar, and Google were searched to determine the presence of Borreliella and Borrelia spp. in vectors, animals, and humans in countries around the Mediterranean Sea. A total of 3026 were identified and screened and after exclusion of papers that did not fulfill the including criteria, 429 were used. After examination of the available literature, it was revealed that various species associated with LD and RF are prevalent in vectors, animals, and humans in Mediterranean countries and should be monitored in order to effectively manage and prevent potential infections.
Keywords: One Health, Lyme Disease, relapsing fever, Borreliella, Borrelia, bacteria, tick-borne, louse-borne, human-animal-environment interaction, zoonoses
1. Introduction
One Health approach is a holistic perspective that deals with complicated issues involving human, animal, and environmental health [1]. Such approaches have become increasingly important in recent years due to critical changes that have altered the interactions among humans, animals, and their environment [2]. Human population growth as well as their expansion into new geographical areas results in closer proximity between people and animals, facilitating the transmission of diseases among them [3,4]. Additionally, climate change and alterations in land use, along with increased movement of people, animals, and animal products through international travel and trade, further exacerbates the spread of endemic diseases [5,6].
Borrelia spp. are microaerophilic, slow-growing spirochetal bacteria that have been associated with human diseases [7]. Depending on the infecting species, it can cause Lyme Disease (LD) [8], also known as Lyme borreliosis, or Relapsing Fever (RF) [9].
LD is the most prevalent vector-borne disease in moderate climates of the northern hemisphere with an increasing incidence [10]. It is estimated that approximately 476,000 cases are diagnosed and treated annually in the United States [11], and over 200,000 cases per year in Europe [12].
RF can be either louse-borne (LBRF) or tick-borne (TBRF) [13] and is predominantly found in Africa, Spain, Saudi Arabia, Asia, and western United States, with epidemics still occurring in developing countries due to poor living conditions, famine, and war [14]. Currently, LBRF is mostly observed in the Horn of Africa and among immigrants originating from endemic areas [15]. TBRF has recently been associated with travel-related disease after travelling in endemic areas [16].
Borrelia spp. can be transmitted by hard and soft ticks, as well as human body lice. The distribution of these arthropod vectors is closely linked to the presence of the bacteria in both animals and humans [17]. Animals serve as hosts for both ticks and bacteria, playing a crucial role in the circulation of Borrelia spp. [18]. Therefore, studying the distribution of ticks in relation to the surrounding environment and the prevalence of suitable hosts for ticks and Borrelia spp. is essential for better understanding the factors contributing to human diseases [19].
The aim of this article is to review the current data on the presence of Borrelia spp. in vectors, animals, and humans around the Mediterranean Sea in order to monitor their human-animal-environment interactions within a One Health approach. Such information provides insight for the better surveillance and management of Borrelia infections.
2. Methods
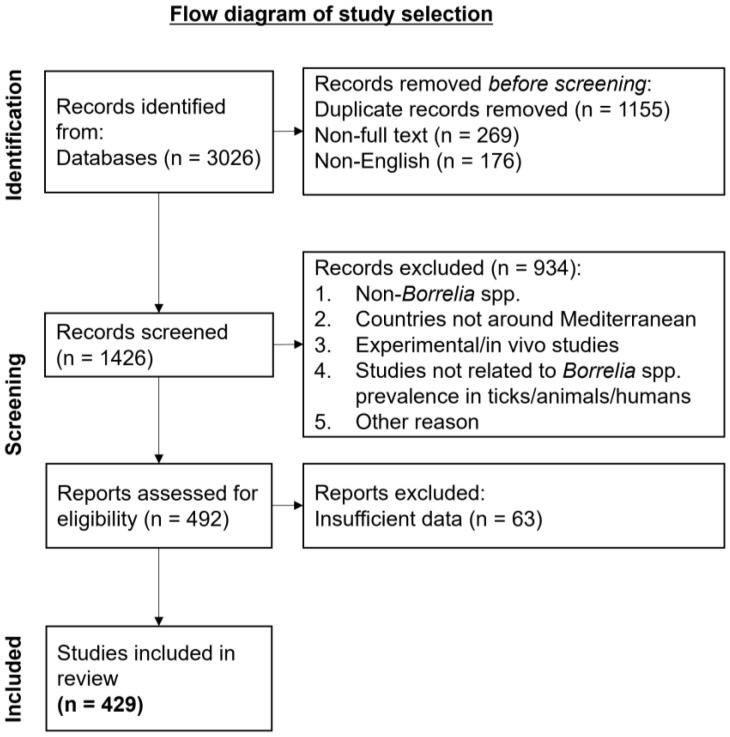
A review of the literature was conducted on the available publications from 1936 to February of 2024 by searching PubMed, Google Scholar, and Google. We included studies related to the presence of LD Borreliella spp. and RF Borrelia spp. in countries around the Mediterranean Sea in the vectors of transmission, the animal hosts, as well as the humans that can become infected. Search terms included “Lyme Disease”, “Lyme borreliosis”, “Relapsing Fever”, “Borreliella”, “Borrelia”, “bacteria”, “human-animal-environment interaction”, “One Health”, “humans”, “animals”, “ticks”, “lice”. Articles that were not written in English were excluded from this study (Figure 1).
Figure 1.
Flow diagram of study selection.
3. Bacteriology
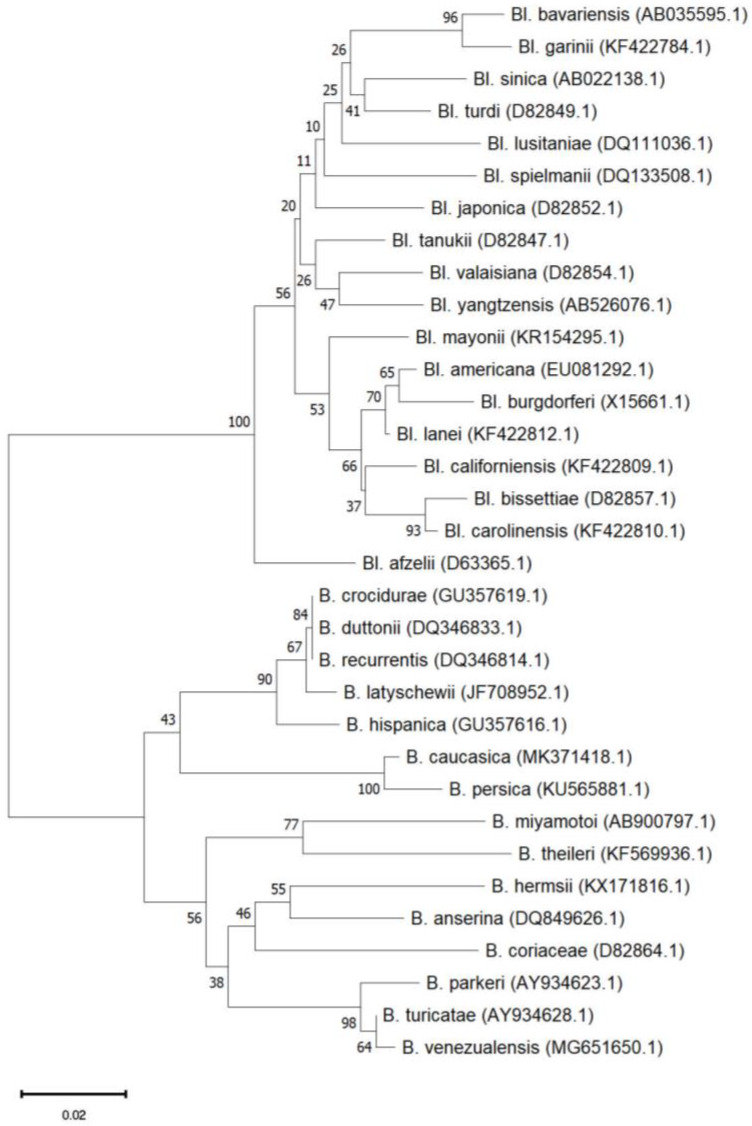
Species of the Borrelia genus are members of the Borreliaceae family of the Spirochaetales phylum and are thus characterized by the typical spirochaete (spiral) shape [20]. They are microaerophilic and host-associated since they rely on their hosts for most of their nutrients in an obligate parasitic lifestyle [21]. They possess an outer membrane around their protoplasmic cylinder analogous to that of Gram-negative bacteria, but without lipopolysaccharides [22]. They have several axial filaments attached to each pole, called endoflagella [13], between the outer membrane and the peptidoglycan layer, inside the periplasmic space, which help the bacteria move forward in a corkscrew-like motion [8]. Their genome consists of one highly conserved linear chromosome and several plasmids that can be either linear or circular and their number varies highly among strains [23]. Borrelia genus is divided into two genera related to human disease, Borreliella for the Lyme group and Borrelia for the Relapsing Fever group (Figure 2). Even though they belong to the same genus, they are known to have significant differences regarding their biological characteristics, their epidemiology, as well as their pathogenicity [24]. According to the phylogenetic data, although they share a genetic similarity, they are different, and they belong to independent clades under a common ancestor. For this reason, it has been proposed and recently validated [25], to include the Lyme group Borrelia into a new genus taxon named Borreliella [26].
Figure 2.
Phylogenetic tree of the genus Borrelia from LD and RF groups constructed with the neighbor joining method with 1000 Bootstrap replications in MEGA 11.0 software based on the flaB gene.
3.1. LD
LD is caused by the Borreliella genospecies. Currently, it contains twenty-six species, twenty-three validated, and three proposed (Table 1) [27,28]. It consists of bacteria 10–20 μm long, approximately 0.3 μm in diameter and the number of their endoflagella ranges from seven to twelve [24]. The species that most commonly cause human disease are Borreliella burgdorferi, Borreliella afzelii, Borreliella garinii, Borreliella mayonii, Borreliella spielmanii, and Borreliella bavariensis. Bl. burgdorferi is responsible for the majority of the LD cases in North America, Bl. garinii in Asia, and Bl. afzelii along with Bl. garinii in Europe [29,30].
Table 1.
Borreliella spp. of the LD Group.
| Borreliella Species | Human Infection |
Geographical Distribution | Hosts | Vectors | References |
|---|---|---|---|---|---|
| Borreliella afzelii | Yes | Europe, Asia | Rodents | I. ricinus, I. persulcatus, I. pavlovsky | [31,32,33,34] |
| Borreliella americana | Unknown | North America, Poland | Rodents, Birds | I. pacificus, I. minor, I. ricinus | [35,36,37] |
| Borreliella andersonii | Unknown | USA | Cottontail rabbits | I. scapularis, I. dentatus | [38,39,40] |
| Borreliella bavariensis | Yes | Europe, Asia | Rodents | I. ricinus, I. persulcatus | [41,42,43] |
| Borreliella bissettiae | Yes | USA, Europe | Rodents | I. spinipalpis, I. ricinus, I. pacificus | [34,44,45,46,47] |
| Borreliella burgdorferi | Yes | America, Europe, Asia | Rodents, Mammals, Birds |
I. scapularis, I. pacificus, I. ricinus, I. kaiseri |
[34,48,49,50] |
| Borreliella californiensis | Unknown | USA, Europe | Kangaroo Rats | I. jellisoni, I. spinipalipis, I. pacificus, I. ricinus | [34,46,51,52,53] |
| Borreliella carolinensis | Unknown | USA, Europe | Rodents | I. minor, I. ricinus, I. affinis | [37,54,55,56,57,58] |
| Borreliella chilensis | Unknown | Chile | Rodents | I. stilesi | [59] |
| Borreliella finlandensis | Unknown | Finland | Unknown | I. ricinus | [60] |
| Borreliella garinii | Yes | Europe, Asia | Rodents, Birds | I. ricinus, I. persulcatus | [34,61] |
| Candidatus Borreliella ibitipoquensis | Unknown | Brazil | Birds | I. paranaensis | [62] |
| Borreliella japonica | Unknown | Japan | Rodents | I. ovatus | [63,64] |
| Borreliella kurtenbachii | Unknown | North America | Rodents | I. scapularis | [58,65,66] |
| Borreliella lanei | Unknown | USA, Europe | Lagomorph | I. spinipalpis, I. pacificus, I. ricinus | [34,44,52,53,67,68] |
| Borreliella lusitaniae | Yes | Europe, North Africa | Lizards, Rodents | I. ricinus | [69,70,71] |
| Borrelia maritima | Unknown | North America | Unknown | Ixodes spinipalpis | [72] |
| Borreliella mayonii | Yes | USA | Rodents | I. scapularis | [73,74,75] |
| Candidatus Borrelia paulista | Unknown | Brazil | Rodents | Unknown | [76] |
| Candidatus Borrelia sibirica | Unknown | Siberia | Rodents | I. apronophorus, I. persulcatus, I. trianguliceps | [77] |
| Borreliella sinica | Unknown | China | Rodents | I. ovatus | [78] |
| Borreliella spielmanii | Yes | Europe | Rodents | I. ricinus | [79,80,81,82] |
| Borreliella tanukii | Unknown | Japan | Unknown | I. tanuki | [83,84,85] |
| Borreliella turdi | Unknown | Japan, Europe | Birds | I. turdus, I. frontalis, I. ricinus | [84,85,86,87] |
| Borreliella valaisiana | No | Europe, Asia | Birds | I. ricinus | [88,89,90,91,92] |
| Borreliella yangtzensis | Unknown | Asia | Rodents | I. granulatus | [93,94,95,96,97] |
3.2. RF
RF is caused by the species of the relapsing fever complex, which consists of 22 validated and several proposed species (Table 2) [98,99]. Bacteria from the RF complex tend to have shorter bodies than the Lyme group and the number of their endoflagella ranges from 15 to 20 [98]. Depending on the vector of transmission, the species of the RF group are categorized in the soft-tick-borne relapsing fever (STBRF), the hard-tick-borne relapsing fever (HTBRF), and the louse-borne relapsing fever (LBRF) group [99]. Additionally, STBRF are further classified according to the geographical area into the Old World strains and the New World strains [100]. Another category is the avian RF which consists of Borrelia anserina and is transmitted by the soft tick Argas spp. [101].
Table 2.
Borrelia spp. of the RF Group.
| Borrelia Species | Human Infection | Geographical Distribution |
Hosts | Vectors | References |
|---|---|---|---|---|---|
| Louse-Borne Relapsing Fever | |||||
| Borrelia recurrentis | LBRF | Virtually worldwide, currently Ethiopia, Sudan | Human | Pediculus humanus | [14,102] |
| Old World Soft-Tick Borne Relapsing Fever | |||||
| Candidatus Borrelia algerica | TBRF | Algeria | Human | Unknown | [103] |
| Borrelia caucasica | STBRF | Azerbaijan, Georgia, Armenia, Ukraine | Rodents, Human | O. verrucosus | [104,105,106,107] |
| Borrelia crocidurae | STBRF | Western and Northern Africa | Insectivores, Rodents, Camels, Human | O. sonrai, O. erraticus | [105,107,108] |
| Borrelia duttonii | STBRF neurological signs, neonatal infection | Central, Eastern, and Southern Africa | Human | O. moubata | [105,107,109,110] |
| Borrelia graingeri | Unknown | East Africa | Rodents | O. graingeri | [105,107,111,112] |
| Borrelia harveyi | Unknown | Kenya | Monkey | Unknown | [105,113,114] |
| Borrelia hispanica | STBRF Ocular and neurological symptoms (rare) | Iberian Peninsula and Northwestern Africa | Insectivores, Rodents, Foxes, Jackals, Dogs, Human | O. erraticus, O. marocanus, O. occidentalis | [105,107,115] |
| Candidatus Borrelia kalaharica | STBRF | Kalahari Desert (Botswana and Namibia) | Human | O. savignyi | [116] |
| Borrelia latyschewii | Unknown | Central Asia, Middle East | Unknown | O. tartakovsky | [105,114,117] |
| Borrelia merionesi | No | Morocco and Atlantic coastal areas of the Sahara Desert | Rodents | O. merionesi, O. costalis, O. erraticus | [118] |
| Borrelia microti | STBRF | Iran, Afghanistan | Human | O. erraticus | [107,119,120,121] |
| Borrelia persica | STBRF, neurological symptoms (rare), respiratory distress (rare) | Central Asia, Middle East, Egypt, Greece | Rodents, Carnivores, Dogs, Cats, Human | O. tholozani | [102,105,122] |
| Borrelia tillae | No | Southern Africa | Rodents | O. zumpti | [123] |
| New World Soft-Tick Borne Relapsing Fever | |||||
| Borrelia brasiliensis | Unknown | Brazil | Unknown | O. brasiliensis | [124,125] |
| Borrelia coriaceae | No | USA | Rodents, Deer | O. coriaceus | [125,126,127,128] |
| Borrelia dugesii | Unknown | Mexico | Unknown | O. dugesii | [105,129] |
| Borrelia hermsii | STBRF, neurological symptoms (rare), neonatal infections (rare) | British Columbia, Western USA, France | Rodents, Deer, Dog, Human | O. hermsi | [102,105,126,128,130] |
| Borrelia johnsonii | TBRF | USA | Bats | O talaje | [131,132,133] |
| Borrelia mazzottii | No | Mexico | Unknown | O. talaje | [134] |
| Borrelia parkeri | Unknown | Western USA | Horses | O. parkeri | [130,135] |
| Borrelia turicatae | STBRF, Ocular and neurological symptoms | British Columbia, USA, Mexico, Africa, Europe, Asia | Rodents, Dog, Human | O. turicata, O. maritimus, C. capensis | [102,105,136] |
| Borrelia venezualensis | STBRF | Central America and northern South America | Human | O. rudis | [105,137] |
| Hard-Tick Borne Relapsing Fever | |||||
| Borrelia lonestari | STARI? | USA | Rodents, Birds, Deer, Human | Amblyomma americanum (lone star tick) | [133,138,139,140,141,142] |
| Borrelia miyamotoi | Flu-like syndrome, HTBRF, neurological symptoms | Asia, Europe, USA | Rodents, Birds, Human | I. ricinus, I. persulcatus, I. scapularis | [143,144,145,146] |
| Candidatus Borrelia texasensis | Unknown | USA (Texas) | Unknown | Dermacentor variabilis | [147] |
| Borrelia theileri | No | Africa, Australia, North and South America | Cattle, Sheep, Goats, Camels | Rhipicephalus spp. | [105,110,148,149] |
| Avian Relapsing Fever | |||||
| Borrelia anserina | No | Worldwide | Birds | Argas spp. | [105,110,150,151,152] |
4. One Health Approach
Global health challenges are escalating, and hence, One Health approaches emerge as a pivotal framework for addressing such complex health matters [153]. This holistic perspective acknowledges the interconnection of these domains and emphasizes the need for collaboration among medical, veterinary, environmental, and other relevant fields [154]. In recent years, the multifaceted nature of Borrelia infections has underlined the necessity for a comprehensive One Health approach. By integrating insights from diverse scientific areas, a One Health perspective offers synergistic efforts to address the complexities of Borrelia infections, enhancing the ability to protect both human and animal populations while promoting environmental sustainability (Figure 3) [155].
Figure 3.
Interactions among humans, animals, and the environment within a One Health concept.
5. Lyme Disease
5.1. Clinical Manifestations
The clinical manifestations of LD can vary widely, as well as the severity and duration of the symptoms, and can affect multiple body systems [156]. The organs most frequently involved are skin, joints, nervous system, heart, and eyes [30,157]. In general, Bl. burgdorferi is more often associated with Lyme arthritis, Bl. garinii with neuroborreliosis, and Bl. afzelii with skin manifestations [30]. The early infection is localized, but the spirochete can spread if left untreated [158]. The most common manifestation of LD that occurs in 80–90% of the cases is a rash known as erythema migrans (EM) [159], which can have divergent characteristics [160,161]. It typically appears as an annular, erythematous rash at the site of the tick bite, which may or may not present with a central clearing [162]. It is a skin redness that appears 1–36 days (with an average of 7–14 days) after the tick bite with a tendency to expand, eventually reaching a diameter of even up to 70 cm (with a median of 10–16 cm) [163,164]. Multiple EM lesions can also occur, with a primary lesion at the site of the tick bite and secondary skin lesions caused by hematogenous dissemination [165]. Additionally, many patients also experience systemic symptoms like fatigue, fever, chills, arthralgia, myalgia, and regional lymphadenopathy [166,167]. Non-cutaneous organ involvement is also possible. Lyme neuroborreliosis occurs in approximately 12% of untreated patients who may present with cranial neuropathy (unilateral or bilateral facial nerve palsy), lymphocytic meningitis, radiculitis, or encephalitis [168,169,170]. Lyme arthritis is the most common late manifestation in the USA, presenting in approximately 60% of untreated patients, but only in a small percentage in Europe [8]. It usually appears months or even up to 2 years after initial infection and typically affects only one or a few large joints, especially the knee [171]. In rare but potentially fatal cases, Lyme carditis appears in 5% of untreated patients usually causing atrioventricular conduction block [172]. The development of chronic congestive cardiomyopathy has also been linked to LD [173,174,175].
5.2. Epidemiology
LD is endemic in temperate regions of the Northern Hemisphere, such as North America, Europe, and Asia [30]. The documented incidence for countries in Europe and Asia varies from negligible in the United Kingdom, Turkey, and Japan to significant in the Netherlands, Belgium, Austria, Slovenia, Lithuania, and Estonia [176,177]. Although incidents have been recorded in countries like China and Mongolia, information on the prevalence of LD in Asia is limited [178,179]. More than 90% of cases in North America are documented from only two regions of the country: the northeast and mid-Atlantic region and the north central region [180]. Both regions have grown significantly over the past 20 years and have extended up to south Canada [181,182]. LD rates are increasing worldwide [181,183] due to increased awareness [184] along with the expanding distribution of the tick vectors due to environmental factors [156,185].
5.2.1. Transmission Vectors Infected with Borreliella spp. in Countries around the Mediterranean Sea
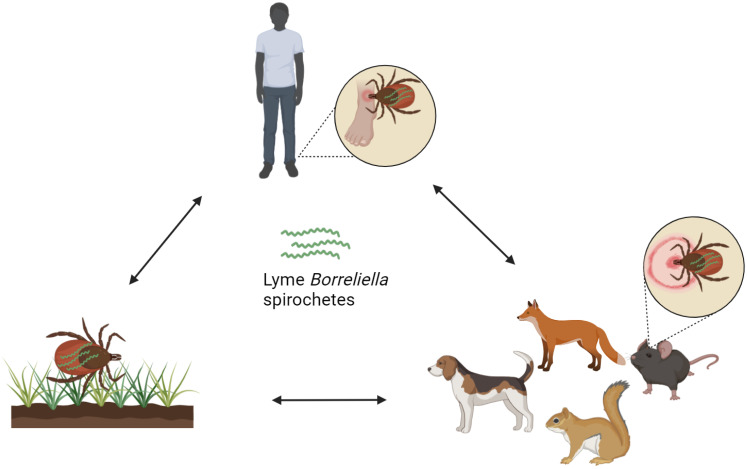
Infectious agents causing LD are transmitted by slow-feeding hard-bodied ticks (ixodid vectors) of the Ixodes ricinus complex and are the only natural agents known through which humans can become infected (Figure 4) [186]. In northeastern, mid-Atlantic, and north central United States, the main vector of transmission is Ixodes scapularis (black-legged tick) and in the Pacific Coast states, Ixodes pacificus [187]. In Asia and in Eastern Europe, the main vector tick is Ixodes persulcatus, while in Western Europe it is Ixodes ricinus [8,188]. Nymphal ticks, rather than the adult ticks, are primarily responsible for transmission of the disease [189]. Spirochaetes are located mainly in the midgut of the tick before attachment and only in small numbers in the salivary glands of the ticks, however, the number in the salivary glands increases rapidly post attachment [190]. Thus, the risk of transmission increases with increased duration of tick attachment and the nymphal ticks are more likely to remain undetected for longer due to their smaller size [191]. The ticks feed on a single vertebrate host when in their larval, nymphal, and adult female phases. Adult male ticks may take sporadic small meals. When a tick, usually a larva or a nymph, feeds on an infected host, the bacteria are acquired by the tick and are subsequently transferred to its midgut [192]. The spirochetes migrate to new hosts, including humans, when the next developing stage of the tick feeds on them [193]. Nevertheless, transovarial transmission which occurs from female to offspring, is rare, resulting in a dead end for the bacteria [194]. A study concerning the human transplacental transmission has been performed, and although Bl. burgdorferi was not directly linked to any of the unfavorable outcomes, the frequency of adverse outcomes emphasizes the need for further studies [195].
Figure 4.
Transmission of Borreliella spirochetes.
Prevalence of the Borreliella complex bacteria have been identified in questing as well as feeding ticks across the Mediterranean. Nonetheless, there are no data on the following Mediterranean countries: Gibraltar, Monaco, Malta, Bosnia and Herzegovina, Montenegro, Albania, Cyprus, Syria, Lebanon, Palestine, Israel, and Libya.
Western Mediterranean (Spain, France)
In Spain, questing I. ricinus ticks were tested for the presence of Borreliella and Bl. garinii was found to be the most prevalent species followed by Bl. valaisiana, Bl. lusitaniae, Bl. afzelii, and Bl. burgdorferi [196]. Additionally, Bl. turdi was found in I. frontalis ticks collected from vegetation of urban and sub-urban areas [197] as well as in I. frontalis, I. ricinus, and Haemaphysalis punctata ticks collected from passerine birds [87]. Moreover, Hyalomma lusitanicum ticks from wild ungulates revealed that they were positive for Bl. afzelii [198]. Borreliella species have also been found in non-Ixodes ticks, as reported in studies with Haemaphysalis spp. ticks, Dermacentor spp., and Rhipicephalus bursa from the vegetation [199,200]. Finally, Borreliella DNA was reported in a male Dermacentor reticulatus removed from a patient [201]. In France, questing I. ricinus ticks from peri-urban forests demonstrated the presence of Bl. afzelii, Bl. burgdorferi, and Bl. garinii followed by Bl. valaisiana, Bl. lusitaniae, and Bl. spielmanii [202]. Moreover, Borreliella carolinensis was reported in one questing I. ricinus tick from a French forest [203]. Furthermore, ticks feeding on humans revealed the presence of Bl. burgdorferi in I. ricinus, and Bl. garinii in I. ricinus and in unidentified Ixodes spp. [204]. Larva ticks engorged on birds were also tested and it was found that Borreliella DNA was present in I. ricinus, I. frontalis, Ixodes spp., and Haemaphysalis spp. [205]. Additionally, I. ricinus ticks from rats and Hyalomma marginatum from cattle from the island of Corsica [206], as well as a questing Dermacentor reticulatus tick from central France were also positive for Borreliella DNA [207].
North Central Mediterranean (Italy, Slovenia)
In Italy, questing I. ricinus ticks, as well as I. ricinus ticks feeding on wild ungulates and domestic animals, were found to be infected with Bl. afzelii, Bl. burgdorferi, Bl. garinii, Bl. valaisiana, and Bl. lusitaniae [208,209]. Moreover, I. ricinus ticks feeding on humans revealed the presence of Borreliella DNA with Bl. afzelii being the most prevalent, followed by Bl. lusitaniae, Bl. garinii, Bl. valaisiana, and Bl. burgdorferi [210]. In addition, Bl. spielmanii and Bl. valaisiana were identified in Ixodes ventalloi and Ixodes acuminatus ticks feeding on feral cats and birds from Pianosa island in the Tuscany archipelago [211], and in Hyalomma rufipes ticks feeding on birds from Ponza island [212]. Likewise, in Slovenia, Bl. garinii was the most predominant species in questing I. ricinus ticks, followed by Bl. afzelii, Bl. valaisiana, Bl. burgdorferi, and Bl. lusitaniae [213]. Finally, Bl. garinii and Bl. valaisiana were found on Ixodes spp. ticks from passerine birds feeding in Slovenia [214].
Balkans (Croatia, Greece)
In Croatia, I. ricinus ticks tested positive for Bl. afzelii, Bl. garinii, Bl. valaisiana, Bl. burgdorferi [215], as well as Bl. lusitaniae [216]. In Greece, Ixodes spp. feeding on birds tested positive for Bl. afzelii and Bl. garinii [214].
Eastern Mediterranean (Turkey)
Questing I. ricinus ticks from Turkey were cultivated leading to the identification of Bl. lusitaniae, Bl. afzelii, Bl. garinii, Bl. burgdorferi, and Bl. valaisiana [217]. Additionally, Bl. burgdorferi was found in Rhipicephalus turanicus collected from wild boar [218], as well as in Hyalomma marginatum, Hyalomma excavatum, Hyalomma spp., and Haemaphysalis parva ticks collected from humans [219]. Borreliella were also prevalent in Rhipicephalus annulatus, in Hyalomma anatolicum, and I. ricinus questing or feeding on humans, pets, or other animals [220].
Southern Mediterranean (Egypt, Tunisia, Algeria, Morocco)
Several tick species feeding on one-humped camels from Cairo, Egypt, demonstrated the presence of Bl. afzelii in Hyalomma dromedarii and H. marginatum, as well as Bl. burgdorferi in H. dromedarii and Amblyomma testudinarium [221]. Moreover, Borreliella were also identified in Rhipicephalus sanguineus from dogs and in Hyalomma anatolicum excavatum from cattle [222]. In Tunisia, questing I. ricinus ticks were positive for Borreliella DNA [223] and after cultivation, Bl. lusitaniae and Bl. garinii were isolated [223,224]. In Algeria, questing I. ricinus ticks were infected with Bl. garinii [225] and I. ricinus ticks feeding on cattle with Bl. lusitaniae [216]. In Morocco, questing I. ricinus ticks were found to be infected with Bl. lusitaniae, Bl. garinii, and Bl. burgdorferi [226].
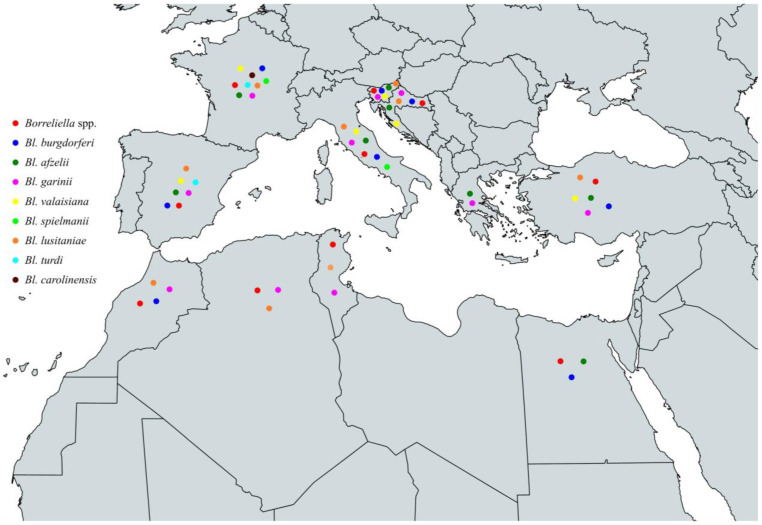
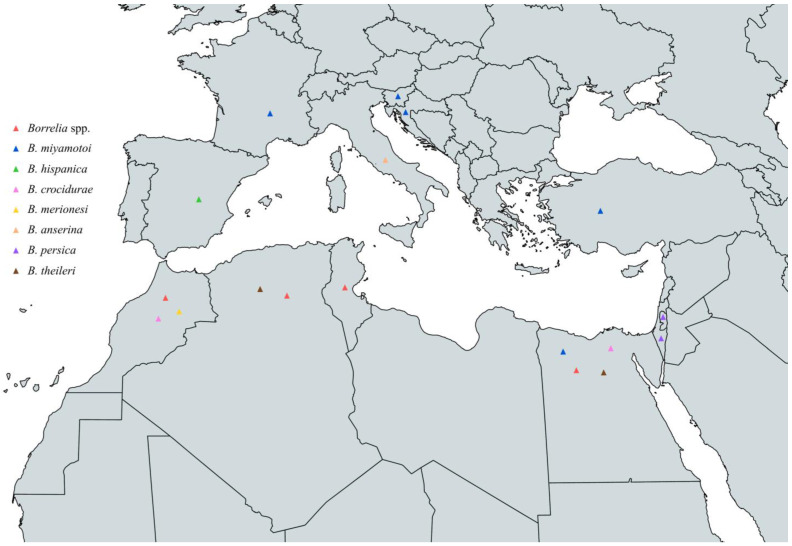
In conclusion, the characterized species of the Borreliella complex that have been identified among ticks throughout the Mediterranean basin are Bl. burgdorferi, Bl. afzelii, Bl. garinii, Bl. valaisiana, Bl. spielmanii, Bl. lusitaniae, Bl. turdi, and Bl. carolinensis. Specifically, Bl. burgdorferi has been identified in Spain, France, Italy, Slovenia, Croatia, Turkey, Egypt, and Morocco, Bl. afzelii in Spain, France, Italy, Slovenia, Croatia, Greece, Turkey, and Egypt, Bl. garinii in Spain, France, Italy, Slovenia, Croatia, Greece, Turkey, Tunisia, Algeria, and Morocco, Bl. valaisiana in Spain, France, Italy, Slovenia, Croatia, and Turkey. Additionally, Bl. spielmanii in France and Italy, Bl. lusitaniae in Spain, France, Italy, Slovenia, Croatia, Turkey, Tunisia, Algeria, and Morocco, Bl. turdi in Spain and France and finally, Bl. carolinensis in France (Figure 5 and Table 3).
Figure 5.
Map of Borreliella spp. in vectors in countries around the Mediterranean Sea.
Table 3.
Borreliella spp. in vectors around the Mediterranean Sea.
| Country | Borreliella Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Borreliella spp. | Bl. burgdorferi | Bl. afzelii | Bl. garinii | Bl. valaisiana | Bl. spielmanii | Bl. lusitaniae | Bl. turdi | Bl. carolinensis | |
| Spain | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| France | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Italy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Slovenia | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Croatia | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Greece | ✓ | ✓ | |||||||
| Turkey | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Egypt | ✓ | ✓ | ✓ | ||||||
| Tunisia | ✓ | ✓ | ✓ | ||||||
| Algeria | ✓ | ✓ | ✓ | ||||||
| Morocco | ✓ | ✓ | ✓ | ✓ | |||||
5.2.2. Animal Hosts Infected with Borreliella spp. in Countries around the Mediterranean Sea
Reservoir hosts are important for the transmission of the bacterium, as they can become heavily infested with ticks and transmit the bacterium to them when they feed on their blood [227]. More than 100 animal species have been identified as Borreliella hosts, including small mammals, birds, and lizards [70,228,229]. The main hosts in North America are small mammals, such as mice, chipmunks, and squirrels, with the white-footed mice being the most common [230,231,232]. In Europe and Asia, the primary hosts are rodents like voles, shrews, and mice like the yellow-necked mice, especially for Bl. afzelii [27,192]. Birds can also become infected with Borreliella and serve as important hosts mainly for Bl. garinii as they can make long-range displacements [233]. Although deer develop antibodies when exposed to Borreliella via tick bites [234], they are incompetent reservoir hosts and do not transmit the infection to feeding ticks [235,236,237].
Infections of the Borreliella complex have been described in several animal species across the Mediterranean. However, there are no data on animal infections and the species involved in the following Mediterranean countries: Gibraltar, Monaco, Malta, Montenegro, Cyprus, Syria, Lebanon, Palestine, Israel, Libya, and Morocco.
Western Mediterranean (Spain, France)
In Spain, Borreliella have been identified in several host species. Wood mice tested positive for Borreliella and voles for Bl. afzelii [199]. Additionally, spleen samples from wild ungulates revealed the prevalence of Bl. afzelii [198]. Antibodies against Borreliella were identified in dogs [238], in red foxes [239], and in wolves [240]. Finally, chamois [241] and roe deer [198] were also presenting antibodies. In France, rodents have been identified as important host species of Borreliella [242,243]. Indeed, Siberian chipmunks were infected with Bl. burgdorferi, Bl. afzelii, Bl. garinii [244], and Bl. spielmanii [245], bank voles with Bl. afzelii, Bl. burgdorferi, and Bl. garinii [245] and wood mice with Bl. afzelii [245]. Additionally, red squirrels have also tested positive for Bl. burgdorferi, Bl. afzelii, and Bl. garinii [246]. Moreover, antibodies against Borreliella have also been identified in dogs [247], horses, and ponies [248]. Last but not least, skin biopsy specimens from deer and roe deer were DNA-positive for Bl. burgdorferi, Bl. garinii or Bl. afzelii, and one Bl. burgdorferi specimen was also positive in culture, indicating the presence of alive spirochetes in deer skin [249].
North Central Mediterranean (Italy, Slovenia)
In Italy, blood samples from rodents were positive for Borreliella DNA [250], and ear biopsies from bank voles and wood or yellow-necked mice revealed the presence of Bl. afzelii, Bl. valaisiana, or Bl. lusitaniae [251]. Foxes were also found to host several Borreliella genospecies, specifically Bl. burgdorferi, Bl. afzelii, Bl. garinii, Bl. valaisiana, Bl. lusitaniae, or Bl. bissettiae, and one fox was co-infected with both Bl. afzelii and Bl. bissettiae [252]. Antibodies against Borreliella were identified in dogs from rural as well as from urban areas [253,254], as well as in horses [255]. Furthermore, Bl. lusitaniae DNA has also been identified in horses [256] and in wall lizards from southern Italy [257]. Besides the animals mentioned above, wild brown hares captured in central Italy were found to carry anti-Borreliella antibodies [258], and one blood sample from a wild boar was positive for Borreliella DNA [259]. Additionally, farm-reared pheasants were seropositive [260] and feral pigeons were positive for the presence of Borreliella DNA [261]. Finally, it was demonstrated that Borreliella DNA was found in blood samples collected from the heart of hunted red deer [262], as well as antibodies against Borreliella in blood samples from fallow deer from farms [263]. In Slovenia, Borreliella DNA was found in heart and lung biopsies from rodents including yellow-necked mice, bank voles, wood mice, Mediterranean water shrews, and a white-toothed shrew [264], and after cultivation, Bl. afzelii was isolated [264]. In addition, antibodies against Borreliella were found in deer [265] and in dogs [266].
Balkans (Croatia, Bosnia and Herzegovina, Albania, Greece)
In Croatia, rodents from eight different collection sites were PCR-positive for Bl. afzelii [267] and a small seropositivity against Borreliella was identified in dogs [266,268,269]. Dogs were also seropositive in Bosnia and Herzegovina against Borreliella [266] and in Albania, for Bl. garinii [270]. In Greece, dogs [271,272], sheep [273], and horses [274] were also found to present antibodies against Borreliella.
Eastern Mediterranean (Turkey)
In Turkey, antibodies against Borreliella were identified in dogs and horses [275] and against Bl. afzelii in mice from North Turkey [276]. Finally, sick house cats tested positive for Borreliella DNA [277].
Southern Mediterranean (Egypt, Tunisia, Algeria)
In Egypt, blood samples from cattle were analyzed and Borreliella DNA was detected [222]. Additionally, dogs were found to have anti-Borreliella antibodies [278], as well as Borreliella DNA [279]. Moreover, Borreliella DNA was also identified in blood samples from healthy one-humped camels [221]. In Tunisia, Borreliella DNA was found in blood samples from goats, sheep, cattle, and camels [280]. Furthermore, lizards were also positive for Bl. lusitaniae as demonstrated by DNA identification from skin, liver, and/or kidney tissue samples, as well as by cultivation of a skin sample [281]. Finally, in Algeria, anti-Borreliella antibodies were found in dogs [282], as well as in horse blood samples [283].
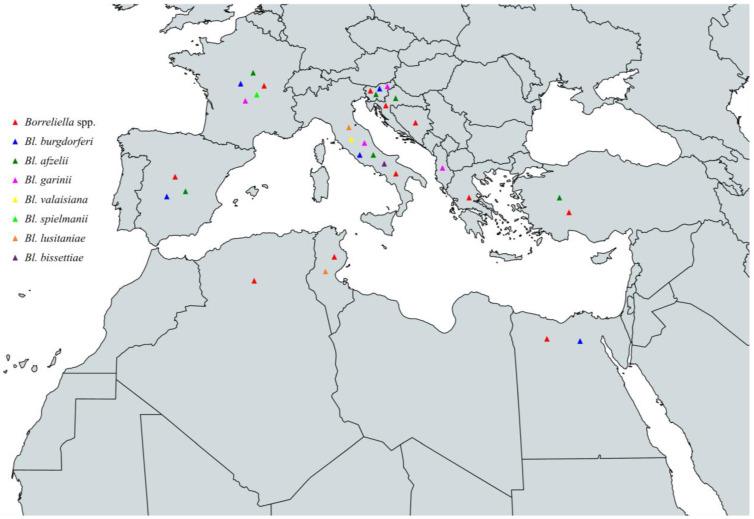
In conclusion, the circulating Borreliella spp. that have been characterized from animals around the Mediterranean are Bl. burgdorferi, Bl. afzelii, Bl. garinii, Bl. valaisiana, Bl. spielmanii, Bl. bissettiae, and Bl. lusitaniae. Specifically, Bl. burgdorferi has been identified in Spain, France, Italy, Slovenia, and Egypt, Bl. afzelii in Spain, France, Italy, Slovenia, Croatia, and Turkey, Bl. garinii in France, Italy, Slovenia, and Albania, Bl. valaisiana in Italy, Bl. spielmanii in France, Bl. bissettiae in Italy, and Bl. lusitaniae in Italy and Tunisia (Figure 6 and Table 4).
Figure 6.
Map of Borreliella spp. in animals in countries around the Mediterranean Sea.
Table 4.
Borreliella spp. in animals around the Mediterranean Sea.
| Country | Borreliella Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Borreliella spp. | Bl. burgdorferi | Bl. afzelii | Bl. garinii | Bl. valaisiana | Bl. spielmanii | Bl. bissettiae | Bl. lusitaniae | |
| Spain |

|

|

|
|||||
| France |

|

|

|

|

|
|||
| Italy |

|

|

|

|

|

|

|
|
| Slovenia |

|

|

|

|
||||
| Croatia |

|

|
||||||
| Bosnia and Herzegovina |

|
|||||||
| Albania |

|
|||||||
| Greece |

|
|||||||
| Turkey |

|

|
||||||
| Egypt |

|

|
||||||
| Tunisia |

|

|
||||||
| Algeria |

|
|||||||
 Rodents
Rodents  Dogs
Dogs  Deer
Deer  Foxes/Wolves
Foxes/Wolves  Goats/Chamois
Goats/Chamois  Horses
Horses  Squirrels/Chipmunks
Squirrels/Chipmunks  Hares
Hares  Birds
Birds  Lizards
Lizards  Sheep
Sheep  Cats
Cats  Cattle
Cattle  Camels
Camels  Wild boar.
Wild boar.
5.2.3. Borreliella spp. in Humans in Countries around the Mediterranean Sea
Many Borreliella infections in humans are reported using molecular and/or serological assays around the Mediterranean and are associated with different genospecies of the Borreliella complex. However, up to date, there are no reports of human infection or very little is known on its prevalence and the species involved in the following Mediterranean countries: Gibraltar, Monaco, Malta, Montenegro, Cyprus, Syria, Lebanon, Palestine, Libya, Tunisia, Algeria, and Morocco.
Western Mediterranean (Spain, France)
In Spain, serum samples from the general population [284] as well as from patients bitten by ticks [201] were found to have antibodies against Bl. burgdorferi using the Bl. burgdorferi B31 strain. Additionally, Bl. garinii was isolated from skin biopsy specimens from patients with EM [285]. In France, synovial fluids were tested for the presence of Borreliella DNA, revealing that the most prevalent species was Bl. burgdorferi, followed by Bl. afzelii and Bl. garinii [286]. Furthermore, skin samples from patients with borrelial lymphocytoma resulted in the molecular identification of Bl. afzelii, Bl. burgdorferi, and Bl. garinii, which was also positive in cultivation [287]. Recently, a case of LD caused by Bl. spielmanii was also reported manifesting as a large EM [288].
North Central Mediterranean (Italy, Slovenia)
Skin biopsies from patients in Italy with clinically diagnosed LD resulted in the isolation of Bl. afzelii, Bl. garinii, and Bl. burgdorferi [289]. Additionally, patients with early or late LD symptoms were found to be infected with Bl. afzelii and Bl. garinii. One co-infection of Bl. garinii with Bl. afzelii and another with Bl. valaisiana were also reported [290]. On the other hand, serum samples from blood donors in South Tyrol revealed the presence of anti-Bl. afzelii and anti-Bl. bavariensis antibodies using Western Blot analysis [291]. Several Borrelia species have also been responsible for human infections in Slovenia. Culture-positive skin samples from patients with only EM as the clinical manifestation of LD indicated that the majority of the infections were due to Bl. afzelii, followed by Bl. garinii and Bl. burgdorferi [292]. Except from the species reported above, in Slovenia, Bl. bavariensis has also been isolated from humans with LD [41,293]. In addition, Bl. spielmanii was also isolated from skin biopsy specimens of patients with EM [294,295]. Moreover, patients with borrelial lymphocytoma were found to be infected by Bl. afzelii, Bl. garinii, or Bl. bissettiae [296].
Balkans (Croatia, Bosnia and Herzegovina, Albania, Greece)
Human infections have also been reported in the Balkan region. In Croatia, antibodies against Bl. burgdorferi (strain B31) were found among various population groups [297]. On the other hand, molecular identification of Bl. afzelii DNA was reported in serum samples from patients with EM from northwest Croatia [298]. Additionally, a woman with EM was found to be infected by Bl. garinii [299]. In Bosnia and Herzegovina, a case of Lyme neuroborreliosis was reported in a male patient two months after a tick bite in a forest from the southern part of the country [300] and, in Albania, the Institute of Public Health reported the diagnosis of LD cases [270] without determination of the specific Borreliella species responsible for the disease. Finally, in Greece, serum samples from patients with suspected zoonotic infection were tested and the presence of anti-Borreliella antibodies was reported [301]. Positive and equivocal samples were further analyzed by Western Blot indicating Bl. afzelii infection [301].
Eastern Mediterranean (Turkey, Israel)
In Turkey, LD cases have been documented [302], as well as seropositivity against Bl. burgdorferi from randomly selected subjects [303] and farmers and forestry workers [304]. A case of LD was also reported in Israel in 1991 [305].
Southern Mediterranean (Egypt)
Finally, in Egypt, people with close contact to feverish cattle and dogs with loss of body weight have been found positive for Borreliella with serological and molecular assays [222].
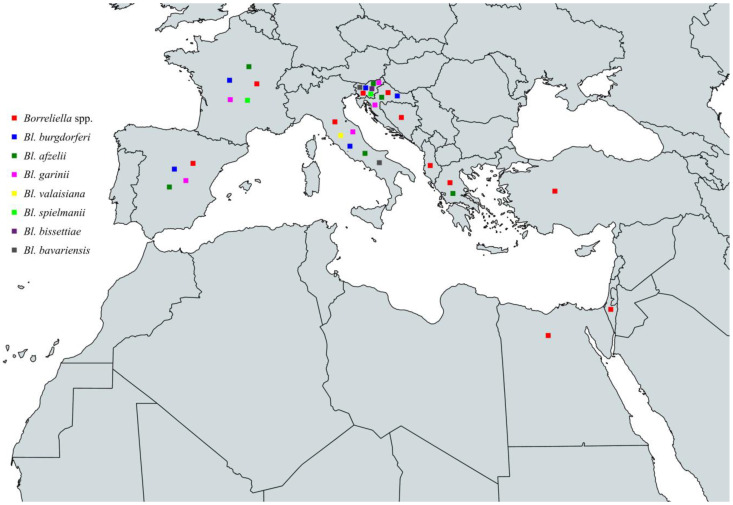
To summarize, the circulating Borreliella spp. from the Borreliella complex that have been characterized from human infections around the Mediterranean Sea are Bl. burgdorferi, Bl. afzelii, Bl. garinii, Bl. valaisiana, Bl. bavariensis, Bl. spielmanii, and Bl. bissettiae. Specifically, Bl. burgdorferi has been reported in Spain, France, Italy, Slovenia, and Croatia, Bl. afzelii in Spain, France, Italy, Slovenia, Croatia, and Greece, Bl. garinii in Spain, France, Italy, Slovenia, and Croatia, Bl. valaisiana in Italy, Bl. bavariensis in Italy and Slovenia, Bl. spielmanii in France and Slovenia, and Bl. bissettiae in Slovenia (Figure 7 and Table 5, Box 1).
Figure 7.
Map of Borreliella spp. in humans in countries around the Mediterranean Sea.
Table 5.
Borreliella spp. in humans around the Mediterranean Sea.
| Country | Borreliella Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Borreliella spp. | Bl. burgdorferi | Bl. afzelii | Bl. garinii | Bl. valaisiana | Bl. bavariensis | Bl. spielmanii | Bl. bissettiae | |
| Spain | ✓ | ✓ | ✓ | ✓ | ||||
| France | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Italy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Slovenia | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Croatia | ✓ | ✓ | ✓ | ✓ | ||||
| Albania | ✓ | |||||||
| Greece | ✓ | ✓ | ||||||
| Turkey | ✓ | |||||||
| Israel | ✓ | |||||||
| Egypt | ✓ | |||||||
Box 1. Prevention of LD.
Spending time in tick infested areas always carries the risk of contracting LD. However, several measures can be implemented to minimize this risk. First, tick repellent should be applied to both clothing and skin before venturing outdoors. It is also important to consistently wear long-sleeved shirts and long trousers, ensuring that they remain tucked into socks throughout the entire duration spent outside. In the case of accompanying pets, acaricides should also be used. During outdoor activi-ties, it is recommended to avoid areas with dense grass and leaf litter. After outdoor activities, a thor-ough examination should be conducted on clothing, gear, and pets for the presence of ticks. Addition-ally, inspection of the human body, including the scalp, is of great importance. If any ticks are found, appropriate removal using tweezers is essential as soon as possible. Finally, modifying the surround-ings of residential areas by clearing leaf litter, placing wood chips in areas where lawns meet forests, applying acaricides, and constructing fences to prevent the entry of tick infested animals is advisable to reduce tick populations.
6. Relapsing Fever
6.1. Clinical Manifestations
RF can be transmitted to humans through the bites of infected soft ticks, hard ticks, or body lice causing STBRF, HTBRF, and LBRF respectively. The clinical manifestations can vary, but RF hallmark is recurring episodes of high fever which can reach as high as 41 °C, spaced by afebrile periods [13]. RF patients usually experience headache, myalgia, arthralgia, and chills. Other symptoms such as fatigue, nausea and vomiting, abdominal pain and diarrhea, skin rash, neurological symptoms such as confusion and disorientation, and cardiac symptoms such as palpitations and chest pain may also occur. The severity of symptoms can vary from almost asymptomatic to lethal, depending on the species of Borrelia involved, the host immune response, and the stage of the infection. Untreated RF can lead to serious complications such as meningitis, hepatitis, and multi-organ failure in all three types of RF.
6.1.1. TBRF
The symptoms of STBRF usually begin approximately one week after the bite of an infected tick, however the incubation period ranges from 4 to 18 days [9]. Patients typically develop an abrupt fever onset of 38.7–40 °C but can reach even up to 41 °C [99]. The first febrile episode is usually the longest and lasts for 12 h to 17 days with a median of 3 days, is frequently accompanied by nonspecific symptoms including headache, arthralgia, myalgia, and nausea, and ends with a crisis of shivering chills or rigors [306,307]. The average time between the first episode and the first relapse is 7 days and the number of recurrences of fever is up to 13 if left untreated [308]. Due to their increased neurotropism, B. duttonii and B. turicatae infections have neurological symptoms that are more severe than those of other RF pathogens, with meningitis and facial palsy among the most prevalent symptoms [102].
Untreated B. duttonii infections have fatality rates that range from 4 to 10%; however, if appropriate antibiotics are given right away, the death rate is less than 2% [307]. Infection during pregnancy is associated with an increased fatality rate in pregnant women, with 10–15% cases of neonatal deaths and a 43.6% perinatal lethality worldwide [309,310].
6.1.2. LBRF
The incubation period is 4–18 days with a median of 7 days [311]. The symptoms initiate suddenly with a fever of almost 40 °C accompanied by stiffness, often followed by headache, anorexia, nausea, vomiting, diarrhea, and generalized pain (muscle, joint, back pain) [311,312]. The length of LBRF episodes ranges from 4 to 10 days, with a median of 5 days, and it takes 5–9 days between the episodes [307,312]. In general, patients with LBRF may have 3 to 5 febrile episodes [308]. Neurological symptoms are less frequent than in TBRF [312]. Fatality rates for untreated disease range from 30 to 80% [313], however, quick administration of the proper antibiotics reduces the fatality rate to 2–5% [306,307]. Infection during pregnancy is associated with adverse outcomes in three out of four pregnancies [313].
6.2. Epidemiology
6.2.1. TBRF
TBRF has a worldwide distribution, with endemic regions in all continents except Australia and Antarctica [16,99]. Over the past few decades, there have been changes in its epidemiology due to the emergence of new TBRF species, the finding of hard tick RF vectors, the extension of the vectors’ geographic range, the improvements in diagnostic techniques, and the increased awareness among healthcare professionals [143,314]. Some areas such as Europe and Japan have developed into endemic areas and in contrast, others like South America and central Africa were once thought to be more endemic than they actually are [9,307]. Additionally, with instances being recorded in people who have visited endemic regions, TBRF has also become recognized as a disease connected to travel [315].
6.2.2. LBRF
The epidemiology of the once-universal LBRF has radically declined during the past few decades [316]. The disappearance of this infection has been directly linked to the decline in the Pediculus humanus humanus lice infestation of clothing, due to improved living standards along with the use of insecticides [317]. LBRF is still endemic almost exclusively in the Horn of Africa [14], especially in Ethiopia [318,319], Somalia [320] and Eritrea [321], and it occasionally spreads to nearby countries like Sudan [308]. Remaining endemic foci of LBRF are also reported to be small parts of Asia [322] and Latin America [323]. Also, in large Mediterranean cities, homeless populations are at risk of re-emerging LBRF following reported huge lice infestation [324,325,326].
6.2.3. Transmission Vectors of Borrelia spp. Identified in Countries around the Mediterranean Sea
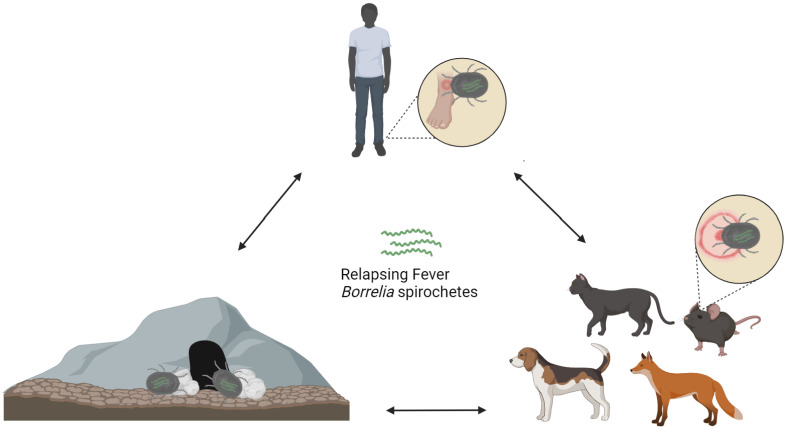
Initially, it was considered that TBRF was transmitted solely by fast-feeding soft-bodied ticks (argasid vectors) of the Ornithodoros genus [107], however, it is now known that Borrelia spp. of the RF group can be transmitted from hard-ticks [143] and human body lice too [327]. In North America, Ornithodoros hermsii and Ornithoodoros turicata are the main tick species to transmit STBRF [306], along with Ornithodoros rudis in South America [328], Ornithodoros erraticus and Ornithodoros tholozani in Eurasia [16,329], and Ornithodoros moubata and Ornithodoros sonrai in Africa [330]. Soft tick bites are rarely noticed since they feed rapidly (15–90 min) and afterwards they return to their habitat. The STBRF-causing spirochetes enter the midgut during the blood meal of Ornithodoros ticks on an infected host, and in the weeks that follow, they colonize the salivary glands where they are maintained transstadially and transovarially [98]. As a result, spirochetes that colonize the salivary gland are more likely to cause an early mammalian infection, leading to rapid transmission of STBRF to the next mammalian host shortly after a bite (Figure 8) [331]. Infection can possibly occur after just 15 s of tick attachment as a tick’s salivary glands are persistently infected [332]. All tick stages are obligate blood feeders and thus potential vectors of Borrelia transmission [306]. Infected ticks remain infectious for their entire lifespan, and they can live up to 10 years infecting several hosts [9,333]. Hard ticks that are known to cause HTBRF are Ixodes spp., Amblyomma spp., Dermacentor spp., and Rhipicephalus spp. [138,334]. The human body louse Pediculus humanus is the only vector of LBRF [316]. Agents of the Borrelia RF group are known to infect tick species throughout the Mediterranean. However, there are insufficient data about Gibraltar, Monaco, Malta, Croatia, Bosnia and Herzegovina, Montenegro, Albania, Greece, Cyprus, Syria, Lebanon, and Libya.
Figure 8.
Transmission of RF Borrelia spirochetes.
Western Mediterranean (Spain, France)
In Spain, DNA from B. miyamotoi was detected in questing I. ricinus nymph and adult ticks [196]. This presence was confirmed across various ecological regions, including coastal, plateau, and mountain areas [335]. Nymph and male I. ricinus ticks feeding on roe deer were also carrying B. miyamotoi bacteria [336]. Additionally, on Espartar Island, a small island in the western Mediterranean Sea, Ornithodoros maritimus ticks from a cave occupied by small seabirds tested positive for Borrelia spp., showing a 99% sequence identity to Borrelia turicatae [337]. In France, DNA samples from free-living I. ricinus nymph ticks collected in the Sénart forest [338] and from the Alpes-Maritimes region [339] revealed the presence of B. miyamotoi. Moreover, B. miyamotoi was prevalent in engorged I. ricinus larva feeding on breeding birds [205] and in I. ricinus and Haemaphysalis punctata ticks feeding on domestic and wild animals from the island of Corsica [340]. Furthermore, Argas vespertilionis ticks collected from a bat-infested attic were also analyzed and a RF Group Borrelia sp. with a 100% sequence identity to strain CPB1 was found [341]. In addition, ticks removed from humans were also tested for the presence of RF Borrelia spp. and it was found that I. ricinus were positive [204,342].
North Central Mediterranean (Italy, Slovenia)
Questing I. ricinus ticks collected by dragging from different altitudes from the mountain areas of northwestern Italy [343], and from both the western and eastern Alps [344], tested positive for B. miyamotoi DNA. B. miyamotoi was also detected in questing I. ricinus ticks from two regions in Slovenia, Littoral Inner Carniola, and the Coastal-Karst area [213].
Eastern Mediterranean (Turkey, Palestine, Israel)
B. miyamotoi DNA was detected in adult I. ricinus ticks collected from the countryside of the European site of Turkey as well as from parks of the metropolitan area of Istanbul [345]. Moreover, Hyalomma aegyptium ticks feeding on Testudo graeca from four different collection sites of Turkey demonstrated the presence of RF Borrelia spp. [346]. Additionally, ticks collected from caves from several locations of the West Bank, Palestine, demonstrated the presence of Borrelia persica in Ornithodoros tholozani [347]. B. persica was also prevalent in nymph female and male O. tholozani ticks from different parts of Israel [348,349].
Southern Mediterranean (Egypt, Tunisia, Algeria, Morocco)
In Egypt, B. miyamotoi was identified in various tick species gathered from one-humped camels in Cairo and Giza. Specifically, these species include Hyalomma dromedarii, Rhipicephalus annulatus, Amblyomma hebraeum, Amblyomma lepidum, Amblyomma cohaerens, and Amblyomma variegatum [221]. Additionally, Borrelia spirochetes were identified in Ornithodoros savignyi from a village of Giza governorate [350]. Furthermore, R. annulatus ticks collected from donkeys tested positive for Borrelia theileri [351], and Ornithodoros erraticus ticks collected from Nile Grass Rat burrows with Borrelia crocidurae [352]. In Tunisia, Rhipicephalus sanguineus ticks feeding on hedgehogs were infected with a RF Group Borrelia sp. [353] and O. erraticus ticks collected from rodent burrows with B. crocidurae [354]. Additionally, B. hispanica was also identified in Ornithodoros sonrai, O. erraticus, and Ornithodoros normandi ticks from small mammal burrows collected from nine different sites [330]. In Algeria, B. crocidurae was also demonstrated in O. sonrai, O. erraticus, and O. normandi ticks from small mammal burrows collected from 18 different sites [330]. In addition, identification of B. hispanica DNA was found in O. occidentalis ticks and B. turicatae in Carios capensis ticks from seabird nests [355]. Furthermore, Argas persicus ticks collected from infested hen farms were also tested and it was revealed that Borrelia anserina was prevalent [356]. Hyalomma aegyptium ticks feeding on Testudo graeca from seven different collection sites of north Algeria as well as from 15 collection sites of central and mostly north Morocco were found to be positive for RF Borrelia spp. [346]. O. sonrai and O. erraticus ticks from small mammal burrows in Morocco were also tested, revealing the presence of B. hispanica, B. crocidurae, and Borrelia merionesi [330,357]. Finally, Ornithodoros marocanus complex ticks from nine sites in Morocco were found infected with B. hispanica and B. crocidurae [358].
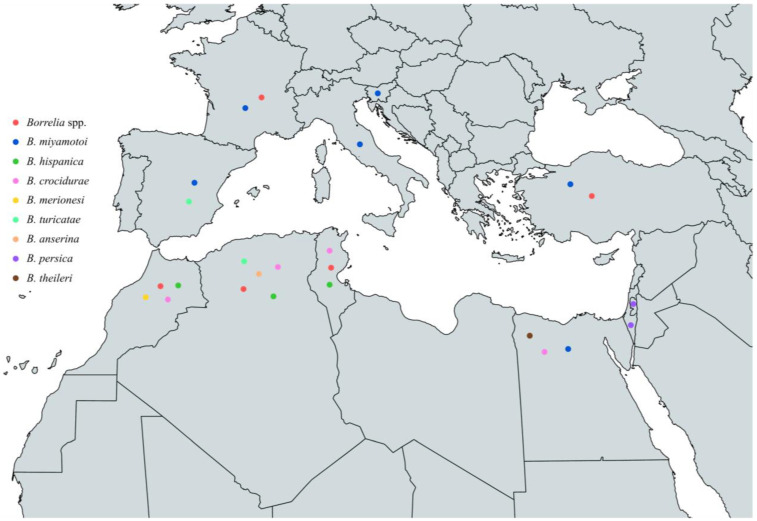
To sum up, the circulating Borrelia spp. that have been identified in vectors around the Mediterranean are B. miyamotoi, B. hispanica, B. crocidurae, B. merionesi, B. turicatae, B. anserina, B. persica, and B. theileri. B. miyamotoi has been reported in Spain, France, Italy, Slovenia, Turkey, and Egypt, B. hispanica in Tunisia, Algeria, and Morocco, and B. crocidurae in Egypt, Tunisia, Algeria, and Morocco. In addition, B. merionesi has been found in ticks in Morocco, B. turicatae in Spain and Algeria, B. anserina in Algeria, B. persica in Palestine and Israel, and B. theileri in Egypt (Figure 9 and Table 6).
Figure 9.
Map of Borrelia spp. in vectors in countries around the Mediterranean Sea.
Table 6.
Borrelia spp. in vectors around the Mediterranean Sea.
| Country | Borrelia Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Borrelia spp. | B. miyamotoi | B. hispanica | B. crocidurae | B. merionesi | B. turicatae | B. anserina | B. persica | B. theileri | |
| Spain | ✓ | ✓ | |||||||
| France | ✓ | ✓ | |||||||
| Italy | ✓ | ||||||||
| Slovenia | ✓ | ||||||||
| Turkey | ✓ | ✓ | |||||||
| Palestine | ✓ | ||||||||
| Israel | ✓ | ||||||||
| Egypt | ✓ | ✓ | ✓ | ||||||
| Tunisia | ✓ | ✓ | ✓ | ||||||
| Algeria | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Morocco | ✓ | ✓ | ✓ | ✓ | |||||
6.2.4. Borrelia spp. in Animals in Countries around the Mediterranean Sea
The hosts of RF bacteria vary depending on the specific species. The majority of species can infect humans and small mammals, and a few species are also able to infect birds, as well as domestic or wild mammals [99]. The main hosts of Borrelia hermsii are small mammals such as chipmunks, squirrels, and mice [359] and of Borrelia miyamotoi are a wide range of mammals, including rodents and birds [145,360]. On the other hand, Borrelia anserina infects exclusively birds and is not associated with human disease [152]. Humans are the primary hosts of Borrelia recurrentis and Borrelia duttonii [102,361,362,363]. Several animal species across the Mediterranean have been found to be infected by species of the Borrelia RF group. Limited data are available, though, for the following Mediterranean countries: Gibraltar, Monaco, Malta, Bosnia and Herzegovina, Montenegro, Albania, Cyprus, Syria, Lebanon, Palestine, and Libya.
Western Mediterranean (Spain, France)
In Spain, B. hispanica has been identified in dogs and a street cat through positive blood smear tests and molecular analysis of 16S rRNA which revealed a 100% similarity with B. hispanica for all samples [364]. In France, research on the prevalence of RF spirochetes in bank voles confirmed the presence of B. miyamotoi [365].
North Central Mediterranean (Italy, Slovenia)
In Italy, pigeons were discovered to have antibodies against B. anserina, after analysis with surface immunofluorescence assay (SIFA) and Western blotting [151]. However, in Slovenia, molecular analysis of rodent biopsy specimens indicated the presence of B. miyamotoi [264].
Balkans (Croatia)
In Croatia, B. miyamotoi has been identified in various rodents, including common shrews, yellow-necked mice, and striped field mice [267].
Eastern Mediterranean (Turkey, Palestine, Israel)
B. miyamotoi has been identified in blood samples from wild rodents from Turkey, also with the highest infection rate in Ural field mice, followed by yellow-necked mice, and bank voles [366]. In addition, in West Bank, Palestine, rodents [349] and rock hyraxes [367] were found to be infected with B. persica. In Israel, several species have been identified as potential hosts for RF Borrelia spp.. Natural infection of B. persica in cats and dogs has been confirmed with positive blood smears and molecular assays [368], as well as with serological assays [369]. Moreover, molecular assays with blood samples from rodents and canids from all over Israel demonstrated that the species most commonly infected with B. persica was the social vole, followed by the red fox, the fat sand rat, the golden jackal, and the Cairo spiny mouse [349]. Additionally, besides golden jackals and red foxes, striped hyenas and European badgers also tested positive for B. persica DNA [370]. Finally, B. persica was also identified in blood and spleen samples from rock hyraxes [367].
Southern Mediterranean (Egypt, Tunisia, Algeria, Morocco)
In Egypt, serum samples from various animals were tested for anti-Borrelia spp. antibodies, with the highest infection rate found in camels, followed by sheep, goats, cows, and buffaloes [350]. Furthermore, blood samples from healthy one-humped camels from Cairo and Giza indicated infections with B. crocidurae and B. miyamotoi [221]. In addition, B. theileri has been identified in cattle [371,372] and in sheep blood [372,373]. In Tunisia, blood samples from fat sand rats were examined microscopically and found to be infected with a Borrelia spp. which could not be further characterized [374]. In Algeria, blood samples from cattle were examined, revealing an infection with RF Borrelia species [375]. Furthermore, B. theileri has also been identified in blood samples from sheep and goats by molecular assays [376]. In Morocco, rodents underwent testing for the presence of Borrelia spp. Thick blood film analysis was conducted in blood samples and molecular assays on brain tissue samples, revealing the presence of spirochetes in both blood and brain tissue samples [330,357]. Positive samples were not further determined to species level, except from one area, where B. merionesi was identified [357]. B. crocidurae has also been reported in rodent and insectivore samples [377].
Concluding, the circulating Borrelia spp. that have been identified in animals across the Mediterranean are B. miyamotoi, B. hispanica, B. persica, B. crocidurae, B. merionesi, B. anserina, and B. theileri. B. miyamotoi has been reported in France, Slovenia, Croatia, Turkey, and Egypt, B. hispanica in Spain, B. persica in Palestine and Israel, B. crocidurae in Egypt and Morocco, B. anserina in Italy, B. merionesi in Morocco, and B. theileri in Egypt and Algeria (Figure 10 and Table 7).
Figure 10.
Map of Borrelia spp. in animals in countries around the Mediterranean Sea.
Table 7.
Borrelia spp. in animals around the Mediterranean Sea.
| Country | Borrelia Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Borrelia spp. | B. hispanica | B. persica | B. crocidurae | B. miyamotoi | B. anserina | B. merionesi | B. theileri | |
| Spain |

|
|||||||
| France |

|
|||||||
| Italy |

|
|||||||
| Slovenia |

|
|||||||
| Croatia |

|
|||||||
| Turkey |

|
|||||||
| Palestine |

|
|||||||
| Israel |

|
|||||||
| Egypt |

|

|

|

|
||||
| Tunisia |

|
|||||||
| Algeria |

|

|
||||||
| Morocco |

|

|

|
|||||
 Dogs
Dogs  Cats
Cats  Rodents
Rodents  Birds
Birds  Hyraxes
Hyraxes  Foxes/Jackals/Hyenas
Foxes/Jackals/Hyenas  Sheep
Sheep  Goats
Goats  Cattle/Buffalos
Cattle/Buffalos  Camels
Camels  Badger.
Badger.
6.2.5. Borrelia spp. in Humans in Countries around the Mediterranean Sea
Infections due to the RF Group Borrelia are also present around the Mediterranean. There are no reports of human infection or very little is known on its prevalence and the species involved in the following Mediterranean countries: Gibraltar, Monaco, Malta, Slovenia, Croatia, Bosnia and Herzegovina, Montenegro, Albania, Turkey, Syria, Lebanon, Palestine, and Tunisia.
Western Mediterranean (Spain, France)
In Spain, RF cases have been documented in individuals within one week after exposure to ticks in mountainous regions [378] or following visits to holiday farms [314], working on a pig farm [314], or among intravenous drug users [379]. Moreover, between 1994 and 2016, numerous cases of RF were diagnosed through direct visualization of spirochetes in blood samples. Some of these samples were forwarded for identification, all of which were identified as B. hispanica [380]. Furthermore, from 2004 to 2015 numerous patients were diagnosed with TBRF based on microscopic analysis of blood or cerebrospinal fluid samples and had clinical data similar to those of a B. hispanica infection [381]. In France, B. miyamotoi and B. hermsii have been identified among patients with symptoms related to a tick bite [382]. In addition, a B. miyamotoi infection was confirmed by serological assays after a B. miyamotoi-infected tick bite [204]. Finally, B. recurrentis infections have been identified in homeless individuals by microimmunofluorescence [324,325] and Western Blotting [324].
North Central Mediterranean (Italy)
In Italy, the first autochthonous case of B. crocidurae infection was documented in a 51-year-old woman from Macedonia who was living in Italy from 15 years old and was diagnosed with meningitis due to B. crocidurae as shown by PCR from CSF specimen after a ten-day history of headache, vertical diplopia, and low-grade fever [383].
Balkans (Greece)
Following a two-week camping excursion on the Greek island of Tilos, during which he explored several caves, a traveler from Belgium reported experiencing recurrent febrile episodes. Upon admission to the hospital, spirochetes were detected on his blood smear, and molecular assays to identify the responsible Borrelia sp. confirmed a B. persica infection [384].
Eastern Mediterranean (Cyprus, Israel)
In Cyprus, a number of cases involving RF among civilians and military personnel, all of whom had somehow spent time in caves or near tick habitats, have been documented over the past century, and in some cases spirochetes were also observed in the blood smears of these individuals as well [385,386]. Also, a case of RF contracted in Cyprus and imported in the United Kingdom was published regarding a soldier who had spent three weeks on exercise in Cyprus and spent the night inside a cave where he observed ticks on his feet when he woke up [387]. In Israel, several cases of RF have been reported during the past years. Outbreaks in 2009 and 2010 have been reported among soldiers during cave entry and prolonged close contact with the ground in the context of a military exercise [388]. Additional cases have been documented, including those diagnosed by a hematological laboratory [389], neonatal cases [390] with severe Jarisch–Herxheimer reaction [391], cases who received placebos in a double-blind trial for post-exposure treatment to prevent TBRF [392], and cases confirmed by molecular assays for B. persica infection [348,393]. B. persica was also identified in spirochetemic patients from Israel as well as in patients with similar clinical manifestations, but with negative blood smears [394], and in a TBRF patient with adult respiratory distress syndrome (ARDS) [395].
Southern Mediterranean (Egypt, Libya, Algeria, Morocco)
A case of RF in a patient with nephritis and subarachnoid hemorrhage, confirmed by blood film, was report in Egypt after spending the night on the ground before the onset of the symptoms [396]. Additionally, humans have been found to carry antibodies against Borrelia spp. antigen [350]. In Libya, cases of RF with confirmed diagnosis by blood film, have been reported in one case of a young Palestinian driver who had not been anywhere else other than Benghazi for at least one month before the onset of his symptoms, an Italian P.O.W. whose specific route before the illness onset is unknown, but he came from Western Cyrenaica, an Indian pioneer who had spent his time wholly in Cyrenaica and in Benghazi, and a British lance-corporal who spent two nights in Tobruk and otherwise was totally in Benghazi [397]. In Algeria, Borrelia spp. DNA has been identified in feverish patients from Oran, and one sample was indicative of a new Borrelia spp. called Candidatus Borrelia algerica [103]. Finally, in Morocco, cases of RF have been confirmed in patients with unexplained fever by positive blood film [357], as well as by molecular assays demonstrating B. hispanica infections [398,399].
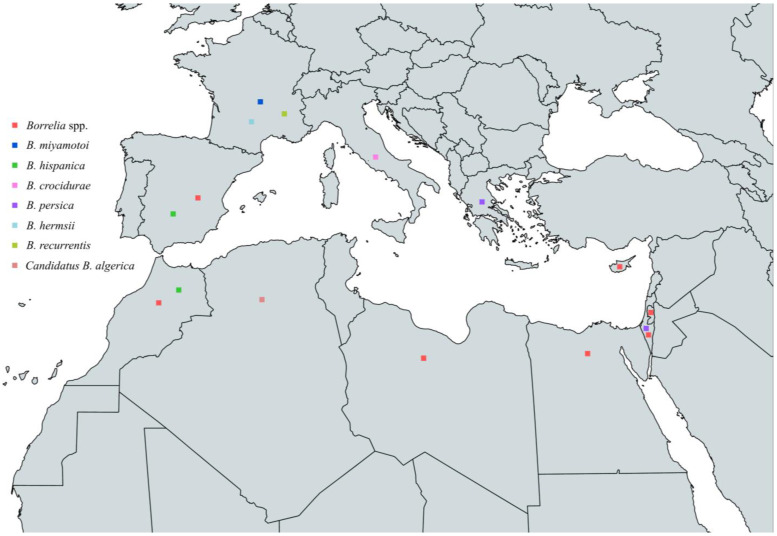
To sum up, the circulating Borrelia spp. that have been reported to cause human infections in countries around the Mediterranean Sea are B. hispanica, B. persica, B. crocidurae, B. miyamotoi, B. hermsii, Candidatus B. algerica, and B. recurrentis. B. hispanica has been reported in Spain and Morocco, B. persica in Greece and Israel, B. crocidurae in Italy, B. miyamotoi in France, B. hermsii in France, Candidatus B. algerica in Algeria, and B. recurrentis in France (Figure 11 and Table 8, Box 2).
Figure 11.
Map of Borrelia spp. in humans in countries around the Mediterranean Sea.
Table 8.
Borrelia spp. in humans around the Mediterranean Sea.
| Country | Borrelia Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Borrelia spp. | B. hispanica | B. persica | B. crocidurae | B. miyamotoi | B. hermsii | Candidatus B. algerica | B. recurrentis | |
| Spain | ✓ | ✓ | ||||||
| France | ✓ | ✓ | ✓ | |||||
| Italy | ✓ | |||||||
| Greece | ✓ | |||||||
| Cyprus | ✓ | |||||||
| Palestine | ✓ | |||||||
| Israel | ✓ | ✓ | ||||||
| Egypt | ✓ | |||||||
| Libya | ✓ | |||||||
| Algeria | ✓ | |||||||
| Morocco | ✓ | ✓ | ||||||
Box 2. Prevention of RF.
Preventing TBRF relies on minimizing exposure to soft ticks. This can be achieved by avoiding habitats where soft ticks are prevalent, such as caves, animal burrows, huts, and cabins. Furthermore, it is recom-mended to use tick repellents on clothing and skin, along with wearing long-sleeved shirts and trousers tucked into socks when engaging in outdoor activities. On the other hand, preventing LBRF primarily de-pends on maintaining personal and clothing hygiene to prevent infestation by body lice.
6.2.6. Imported Human Cases of RF in Mediterranean Countries
Cases of RF are not only contracted in Mediterranean countries but can also be transferred there due to travelling or immigration in other countries, especially from central Africa and Asia. In France, several cases of B. crocidurae infection have been identified, most of which were in immigrants from Somalia [400,401,402,403] passing through Mauritania [315] or immigrants from Mali [315]. Additionally, a case of B. hispanica was identified after travel in Morocco and Spain [315] and another of B. persica after a trip to Uzbekistan and Tajikistan [404]. In Italy, the vast majority of imported RF cases are due to B. recurrentis in immigrants from the Horn of Africa, e.g., Somalia, Eritrea, and Ethiopia [15,405,406]. The immigration route usually includes more or less prolonged stays in Sudan and Libya [326,407,408], and in some cases the immigration route includes Kenya and South Sudan, before crossing Sudan and Libya [409,410]. However, a case of B. recurrentis has also been reported in an immigrant from Mali, who passed by Algeria and Libya before arriving to Italy [411]. Also, a B. crocidurae infection was identified in Italy after travel to Senegal [412]. Finally, B. recurrentis infection has been demonstrated in Israel in Ethiopian Pilgrims [413] and in Libya and Italy in immigrants from Somalia with Germany as their final destination, who were passing through Libya and Italy as intermediate stations [14] (Table 9).
Table 9.
Imported RF cases in Mediterranean countries.
| Country of Manifestation | Country of Origin/Immigration Route | Borrelia Species | References |
|---|---|---|---|
| France | Senegal | B. crocidurae | [400,401,402,403] |
| Senegal and Mauritania | B. crocidurae | [315] | |
| Mali | B. crocidurae | [315] | |
| Morocco and Spain | B. hispanica | [315] | |
| Uzbekistan and Tajikistan | B. persica | [404] | |
| Italy | Eritrea/Somalia/Ethiopia and Libya | B. recurrentis | [15] |
| Somalia and Libya | B. recurrentis | [326,408] | |
| Somalia, Kenya, South Sudan, Sudan, and Libya | B. recurrentis | [409] | |
| Somalia/Sudan and Libya | B. recurrentis | [407] | |
| East Africa | B. recurrentis | [405] | |
| Mali, Algeria, and Libya | B. recurrentis | [411] | |
| Somalia, Kenya, Uganda, Sudan, and Libya | B. recurrentis | [410] | |
| Somalia | B. recurrentis | [406] | |
| Senegal | B. crocidurae | [412] | |
| Israel | Ethiopia | B. recurrentis | [413] |
| Libya and Italy as intermediate destinations | Somalia, Ethiopia, Kenya, South Sudan, and Sudan | B. recurrentis | [14] |
7. Conclusions
In order to successfully monitor and limit the spread of Borrelia infections, it is vital that the medical, veterinary, and environmental sectors collaborate. For this reason, we provide insight for every country around the Mediterranean Sea with available information regarding the prevalence of all Borrelia spp. that have been implicated in tick, animal, and human infection. In summary, LD agents were mostly found in the European part of the Mediterranean. Several Borrelia spp. were identified in hard ticks and in numerous animals, such as rodents, wild ungulates, farm animals, and pets. RF agents were identified all around the Mediterranean in both soft ticks as well as hard ticks. Many animal species were reported to be infected with RF agents, such as rodents, pets, and farm animals. In conclusion, infections caused by Borrelia spp. are still a serious issue that afflict thousands of people every year and hence demand our undivided attention.
Supplementary Materials
The tables with all the references of the Borrelia spp. in humans, animals and vectors can be downloaded at https://www.mdpi.com/article/10.3390/pathogens13060512/s1, Table S1: Lyme Group Borreliella spp. in humans [41,201,222,265,270,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,382,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480,481,482,483,484,485,486,487,488,489,490,491,492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517]; Table S2: Lyme Group Borreliella spp. in animals [198,199,221,222,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,365,373,447,496,502,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538]; Table S3: Lyme Group Borreliella spp. in ticks [61,87,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,233,244,250,251,257,259,279,281,285,291,295,335,336,338,339,340,342,343,346,365,447,473,495,501,502,525,535,539,540,541,542,543,544,545,546,547,548,549,550,551,552,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573,574,575,576,577,578,579,580,581,582,583,584,585,586,587,588,589,590,591,592,593,594,595,596,597,598,599,600,601,602,603,604,605,606,607,608,609,610,611,612,613,614,615,616,617,618,619,620,621,622,623,624,625,626,627,628,629,630,631,632,633,634,635,636,637,638,639,640,641]; Table S4: Relapsing Fever Group Borrelia spp. in humans [103,204,314,324,325,347,348,350,357,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396,397,398,399,642,643,644,645,646,647,648,649,650,651]; Table S5: Relapsing Fever Group Borrelia spp. in animals [151,221,264,267,330,349,350,357,364,365,366,367,368,369,370,371,372,373,374,375,376,377,652,653]; Table S6: Relapsing Fever Group Borrelia spp. in ticks [196,204,205,213,221,330,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,351,352,353,354,355,356,357,358,365,447,540,551,559,562,563,566,568,570,575,576,582,643,653,654,655].
Author Contributions
Data curation, M.K.; writing—original draft preparation, M.K.; review and editing, M.D.; supervision, E.A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Calistri P., Iannetti S., Danzetta M.L., Narcisi V., Cito F., Sabatino D.D., Bruno R., Sauro F., Atzeni M., Carvelli A., et al. The components of ‘One World—One Health’ approach. Transbound. Emerg. Dis. 2013;60((Suppl. 2)):4–13. doi: 10.1111/tbed.12145. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie J.S., Jeggo M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019;4:88. doi: 10.3390/tropicalmed4020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinowitz P.M., Kock R., Kachani M., Kunkel R., Thomas J., Gilbert J., Wallace R., Blackmore C., Wong D., Karesh W., et al. Toward proof of concept of a one health approach to disease prediction and control. Emerg. Infect. Dis. 2013;19:e1302650. doi: 10.3201/eid1912.130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karesh W.B., Dobson A., Lloyd-Smith J.O., Lubroth J., Dixon M.A., Bennett M., Aldrich S., Harrington T., Formenty P., Loh E.H., et al. Ecology of zoonoses: Natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones B.A., Grace D., Kock R., Alonso S., Rushton J., Said M.Y., McKeever D., Mutua F., Young J., McDermott J., et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oppler Z.J., O’Keeffe K.R., McCoy K.D., Brisson D. Evolutionary Genetics of Borrelia. Curr. Issues Mol. Biol. 2021;42:97–112. doi: 10.21775/cimb.042.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steere A.C., Strle F., Wormser G.P., Hu L.T., Branda J.A., Hovius J.W., Li X., Mead P.S. Lyme borreliosis. Nat. Rev. Dis. Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakab A., Kahlig P., Kuenzli E., Neumayr A. Tick borne relapsing fever—A systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 2022;16:e0010212. doi: 10.1371/journal.pntd.0010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olafsdottir B., Askling H.H. Increasing spread of borreliosis in Europe. New Microbes New Infect. 2022;48:101022. doi: 10.1016/j.nmni.2022.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kugeler K.J., Schwartz A.M., Delorey M.J., Mead P.S., Hinckley A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021;27:616–619. doi: 10.3201/eid2702.202731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques A.R., Strle F., Wormser G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021;27:2017–2024. doi: 10.3201/eid2708.204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenfeld O. BORRELIA: Strains, Vectors, Human and Animal Borreliosis. CABI Digital Library; Wallingford, UK: 1971. [Google Scholar]
- 14.Hoch M., Wieser A., Loscher T., Margos G., Purner F., Zuhl J., Seilmaier M., Balzer L., Guggemos W., Rack-Hoch A., et al. Louse-borne relapsing fever (Borrelia recurrentis) diagnosed in 15 refugees from northeast Africa: Epidemiology and preventive control measures, Bavaria, Germany, July to October 2015. Eurosurveillance. 2015;20:30046. doi: 10.2807/1560-7917.ES.2015.20.42.30046. [DOI] [PubMed] [Google Scholar]
- 15.Antinori S., Mediannikov O., Corbellino M., Raoult D. Louse-borne relapsing fever among East African refugees in Europe. Travel. Med. Infect. Dis. 2016;14:110–114. doi: 10.1016/j.tmaid.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Rebaudet S., Parola P. Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunol. Med. Microbiol. 2006;48:11–15. doi: 10.1111/j.1574-695X.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurokawa C., Lynn G.E., Pedra J.H.F., Pal U., Narasimhan S., Fikrig E. Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol. 2020;18:587–600. doi: 10.1038/s41579-020-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helble J.D., McCarthy J.E., Hu L.T. Interactions between Borrelia burgdorferi and its hosts across the enzootic cycle. Parasite Immunol. 2021;43:e12816. doi: 10.1111/pim.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couret J., Schofield S., Narasimhan S. The environment, the tick, and the pathogen—It is an ensemble. Front. Cell. Infect. Microbiol. 2022;12:1049646. doi: 10.3389/fcimb.2022.1049646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta R.S., Mahmood S., Adeolu M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: Proposal for a taxonomic revision of the phylum. Front. Microbiol. 2013;4:217. doi: 10.3389/fmicb.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilly K., Rosa P.A., Stewart P.E. Biology of infection with Borrelia burgdorferi. Infect. Dis. Clin. N. Am. 2008;22:217–234. doi: 10.1016/j.idc.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyde J.A. Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion. Front. Immunol. 2017;8:114. doi: 10.3389/fimmu.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz I., Margos G., Casjens S.R., Qiu W.G., Eggers C.H. Multipartite Genome of Lyme Disease Borrelia: Structure, Variation and Prophages. Curr. Issues Mol. Biol. 2021;42:409–454. doi: 10.21775/cimb.042.409. [DOI] [PubMed] [Google Scholar]
- 24.Trevisan G., Cinco M., Trevisini S., di Meo N., Chersi K., Ruscio M., Forgione P., Bonin S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology. 2021;10:1036. doi: 10.3390/biology10101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arahal D.R., Bull C.T., Busse H.J., Christensen H., Chuvochina M., Dedysh S.N., Fournier P.E., Konstantinidis K.T., Parker C.T., Rossello-Mora R., et al. Judicial Opinions 123–127. Int. J. Syst. Evol. Microbiol. 2022;72:005708. doi: 10.1099/ijsem.0.005708. [DOI] [PubMed] [Google Scholar]
- 26.Adeolu M., Gupta R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex) Antonie Leeuwenhoek. 2014;105:1049–1072. doi: 10.1007/s10482-014-0164-x. [DOI] [PubMed] [Google Scholar]
- 27.Steinbrink A., Brugger K., Margos G., Kraiczy P., Klimpel S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022;121:781–803. doi: 10.1007/s00436-022-07445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolcott K.A., Margos G., Fingerle V., Becker N.S. Host association of Borrelia burgdorferi sensu lato: A review. Ticks Tick-Borne Dis. 2021;12:101766. doi: 10.1016/j.ttbdis.2021.101766. [DOI] [PubMed] [Google Scholar]
- 29.Branda J.A., Steere A.C. Laboratory Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 2021;34:e00018-19. doi: 10.1128/CMR.00018-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 31.Canica M.M., Nato F., du Merle L., Mazie J.C., Baranton G., Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 32.Nakao M., Miyamoto K., Fukunaga M., Hashimoto Y., Takahashi H. Comparative studies on Borrelia afzelii isolated from a patient of Lyme disease, Ixodes persulcatus ticks, and Apodemus speciosus rodents in Japan. Microbiol. Immunol. 1994;38:413–420. doi: 10.1111/j.1348-0421.1994.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 33.Korenberg E.I., Nefedova V.V., Romanenko V.N., Gorelova N.B. The tick Ixodes pavlovskyi as a host of spirochetes pathogenic for humans and its possible role in the epizootiology and epidemiology of borrelioses. Vector Borne Zoonotic Dis. 2010;10:453–458. doi: 10.1089/vbz.2009.0033. [DOI] [PubMed] [Google Scholar]
- 34.Wodecka B., Michalik J., Grochowalska R. Red Foxes (Vulpes vulpes) Are Exposed to High Diversity of Borrelia burgdorferi Sensu Lato Species Infecting Fox-Derived Ixodes Ticks in West-Central Poland. Pathogens. 2022;11:696. doi: 10.3390/pathogens11060696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudenko N., Golovchenko M., Lin T., Gao L., Grubhoffer L., Oliver J.H., Jr. Delineation of a new species of the Borrelia burgdorferi Sensu Lato Complex, Borrelia americana sp. nov. J. Clin. Microbiol. 2009;47:3875–3880. doi: 10.1128/JCM.01050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott J.D., Foley J.E. Detection of Borrelia americana in the Avian Coastal Tick, Ixodes auritulus (Acari: Ixodidae), Collected from a Bird Captured in Canada. Open J. Anim. Sci. 2016;6:207–216. doi: 10.4236/ojas.2016.63027. [DOI] [Google Scholar]
- 37.Dunaj J., Drewnowska J., Moniuszko-Malinowska A., Swiecicka I., Pancewicz S. First metagenomic report of Borrelia americana and Borrelia carolinensis in Poland—A preliminary study. Ann. Agric. Environ. Med. 2021;28:49–55. doi: 10.26444/aaem/118134. [DOI] [PubMed] [Google Scholar]
- 38.Anderson J.F., Magnarelli L.A., McAninch J.B. New Borrelia burgdorferi antigenic variant isolated from Ixodes dammini from upstate New York. J. Clin. Microbiol. 1988;26:2209–2212. doi: 10.1128/jcm.26.10.2209-2212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marconi R.T., Liveris D., Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: Phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J. Clin. Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamer S.A., Hickling G.J., Keith R., Sidge J.L., Walker E.D., Tsao J.I. Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasit Vectors. 2012;5:231. doi: 10.1186/1756-3305-5-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margos G., Vollmer S.A., Cornet M., Garnier M., Fingerle V., Wilske B., Bormane A., Vitorino L., Collares-Pereira M., Drancourt M., et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 2009;75:5410–5416. doi: 10.1128/AEM.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margos G., Wilske B., Sing A., Hizo-Teufel C., Cao W.C., Chu C., Scholz H., Straubinger R.K., Fingerle V. Borrelia bavariensis sp. nov. is widely distributed in Europe and Asia. Int. J. Syst. Evol. Microbiol. 2013;63:4284–4288. doi: 10.1099/ijs.0.052001-0. [DOI] [PubMed] [Google Scholar]
- 43.Margos G., Fingerle V., Reynolds S. Borrelia bavariensis: Vector Switch, Niche Invasion, and Geographical Spread of a Tick-Borne Bacterial Parasite. Front. Ecol. Evol. 2019;7:401. doi: 10.3389/fevo.2019.00401. [DOI] [Google Scholar]
- 44.Postic D., Ras N.M., Lane R.S., Hendson M., Baranton G. Expanded diversity among Californian borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J. Clin. Microbiol. 1998;36:3497–3504. doi: 10.1128/JCM.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider B.S., Zeidner N.S., Burkot T.R., Maupin G.O., Piesman J. Borrelia isolates in Northern Colorado identified as Borrelia bissettii. J. Clin. Microbiol. 2000;38:3103–3105. doi: 10.1128/JCM.38.8.3103-3105.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margos G., Lane R.S., Fedorova N., Koloczek J., Piesman J., Hojgaard A., Sing A., Fingerle V. Borrelia bissettiae sp. nov. and Borrelia californiensis sp. nov. prevail in diverse enzootic transmission cycles. Int. J. Syst. Evol. Microbiol. 2016;66:1447–1452. doi: 10.1099/ijsem.0.000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fedorova N., Kleinjan J.E., James D., Hui L.T., Peeters H., Lane R.S. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick-Borne Dis. 2014;5:951–961. doi: 10.1016/j.ttbdis.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Burgdorfer W., Barbour A.G., Hayes S.F., Benach J.L., Grunwaldt E., Davis J.P. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 49.Johnson R.C., Schmid G.P., Hyde F.W., Steigerwalt A.G., Brenner D.J. Borrelia burgdorferi sp. nov.: Etiologic Agent of Lyme Disease. Int. J. Syst. Bacteriol. 1984;34:496–497. doi: 10.1099/00207713-34-4-496. [DOI] [Google Scholar]
- 50.Kim C.M., Park S.Y., Kim D.M., Park J.W., Chung J.K. First report of Borrelia burgdorferi sensu stricto detection in a commune genospecies in Apodemus agrarius in Gwangju, South Korea. Sci. Rep. 2021;11:18199. doi: 10.1038/s41598-021-97411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postic D., Garnier M., Baranton G. Multilocus sequence analysis of atypical Borrelia burgdorferi sensu lato isolates—Description of Borrelia californiensis sp. nov., and genomospecies 1 and 2. Int. J. Med. Microbiol. IJMM. 2007;297:263–271. doi: 10.1016/j.ijmm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Wodecka B., Kolomiiets V. Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland. Life. 2023;13:972. doi: 10.3390/life13040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michalik J., Wodecka B., Liberska J., Dabert M., Postawa T., Piksa K., Stanczak J. Diversity of Borrelia burgdorferi sensu lato species in Ixodes ticks (Acari: Ixodidae) associated with cave-dwelling bats from Poland and Romania. Ticks Tick-Borne Dis. 2020;11:101300. doi: 10.1016/j.ttbdis.2019.101300. [DOI] [PubMed] [Google Scholar]
- 54.Rudenko N., Golovchenko M., Grubhoffer L., Oliver J.H., Jr. Borrelia carolinensis sp. nov., a new (14th) member of the Borrelia burgdorferi Sensu Lato complex from the southeastern region of the United States. J. Clin. Microbiol. 2009;47:134–141. doi: 10.1128/JCM.01183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foley J., Ott-Conn C., Worth J., Poulsen A., Clifford D. An Ixodes minor and Borrelia carolinensis enzootic cycle involving a critically endangered Mojave Desert rodent. Ecol. Evol. 2014;4:576–581. doi: 10.1002/ece3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Springer A., Raulf M.K., Fingerle V., Strube C. Borrelia prevalence and species distribution in ticks removed from humans in Germany, 2013–2017. Ticks Tick-Borne Dis. 2020;11:101363. doi: 10.1016/j.ttbdis.2019.101363. [DOI] [PubMed] [Google Scholar]
- 57.Rudenko N., Golovchenko M., Grubhoffer L., Oliver J.H. Borrelia carolinensis sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex isolated from rodents and a tick from the south-eastern USA. Int. J. Syst. Evol. Microbiol. 2011;61:381–383. doi: 10.1099/ijs.0.021436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osikowicz L.M., Rizzo M.R., Hojgaard A., Maes S.E., Eisen R.J. Detection of Borrelia burgdorferi sensu lato species in host-seeking Ixodes species ticks in the United States. Ticks Tick-Borne Dis. 2024;15:102270. doi: 10.1016/j.ttbdis.2023.102270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivanova L.B., Tomova A., Gonzalez-Acuna D., Murua R., Moreno C.X., Hernandez C., Cabello J., Cabello C., Daniels T.J., Godfrey H.P., et al. Borrelia chilensis, a new member of the Borrelia burgdorferi sensu lato complex that extends the range of this genospecies in the Southern Hemisphere. Environ. Microbiol. 2014;16:1069–1080. doi: 10.1111/1462-2920.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casjens S.R., Fraser-Liggett C.M., Mongodin E.F., Qiu W.G., Dunn J.J., Luft B.J., Schutzer S.E. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J. Bacteriol. 2011;193:1489–1490. doi: 10.1128/JB.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J.C., Assous M., Grimont P.A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 62.Munoz-Leal S., Ramirez D.G., Luz H.R., Faccini J.L.H., Labruna M.B. “Candidatus Borrelia ibitipoquensis”, a Borrelia valaisiana-Related Genospecies Characterized from Ixodes paranaensis in Brazil. Microb. Ecol. 2020;80:682–689. doi: 10.1007/s00248-020-01512-x. [DOI] [PubMed] [Google Scholar]
- 63.Kawabata H., Masuzawa T., Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol. Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 64.Masuzawa T., Suzuki H., Kawabata H., Ishiguro F., Takada N., Yano Y., Yanagihara Y. Identification of spirochetes isolated from wild rodents in Japan as Borrelia japonica. J. Clin. Microbiol. 1995;33:1392–1394. doi: 10.1128/jcm.33.5.1392-1394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margos G., Hojgaard A., Lane R.S., Cornet M., Fingerle V., Rudenko N., Ogden N., Aanensen D.M., Fish D., Piesman J. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick-Borne Dis. 2010;1:151–158. doi: 10.1016/j.ttbdis.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Margos G., Piesman J., Lane R.S., Ogden N.H., Sing A., Straubinger R.K., Fingerle V. Borrelia kurtenbachii sp. nov., a widely distributed member of the Borrelia burgdorferi sensu lato species complex in North America. Int. J. Syst. Evol. Microbiol. 2014;64:128–130. doi: 10.1099/ijs.0.054593-0. [DOI] [PubMed] [Google Scholar]
- 67.Lane R.S., Quistad G.B. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis) J. Parasitol. 1998;84:29–34. doi: 10.2307/3284524. [DOI] [PubMed] [Google Scholar]
- 68.Margos G., Fedorova N., Kleinjan J.E., Hartberger C., Schwan T.G., Sing A., Fingerle V. Borrelia lanei sp. nov. extends the diversity of Borrelia species in California. Int. J. Syst. Evol. Microbiol. 2017;67:3872–3876. doi: 10.1099/ijsem.0.002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Fleche A., Postic D., Girardet K., Peter O., Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 70.Richter D., Matuschka F.R. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl. Environ. Microbiol. 2006;72:4627–4632. doi: 10.1128/AEM.00285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Carvalho I.L., Zeidner N., Ullmann A., Hojgaard A., Amaro F., Ze-Ze L., Alves M.J., de Sousa R., Piesman J., Nuncio M.S. Molecular characterization of a new isolate of Borrelia lusitaniae derived from Apodemus sylvaticus in Portugal. Vector Borne Zoonotic Dis. 2010;10:531–534. doi: 10.1089/vbz.2008.0210. [DOI] [PubMed] [Google Scholar]
- 72.Margos G., Fedorova N., Becker N.S., Kleinjan J.E., Marosevic D., Krebs S., Hui L., Fingerle V., Lane R.S. Borrelia maritima sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex, occupying a basal position to North American species. Int. J. Syst. Evol. Microbiol. 2020;70:849–856. doi: 10.1099/ijsem.0.003833. [DOI] [PubMed] [Google Scholar]
- 73.Johnson T.L., Graham C.B., Hojgaard A., Breuner N.E., Maes S.E., Boegler K.A., Replogle A.J., Kingry L.C., Petersen J.M., Eisen L., et al. Isolation of the Lyme Disease Spirochete Borrelia mayonii From Naturally Infected Rodents in Minnesota. J. Med. Entomol. 2017;54:1088–1092. doi: 10.1093/jme/tjx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pritt B.S., Mead P.S., Johnson D.K.H., Neitzel D.F., Respicio-Kingry L.B., Davis J.P., Schiffman E., Sloan L.M., Schriefer M.E., Replogle A.J., et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect. Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siy P.N., Larson R.T., Zembsch T.E., Lee X., Paskewitz S.M. High Prevalence of Borrelia mayonii (Spirochaetales: Spirochaetaceae) in Field-Caught Tamias striatus (Rodentia: Sciuridae) From Northern Wisconsin. J. Med. Entomol. 2021;58:2504–2507. doi: 10.1093/jme/tjab102. [DOI] [PubMed] [Google Scholar]
- 76.Weck B.C., Serpa M.C.A., Labruna M.B., Munoz-Leal S. A Novel Genospecies of Borrelia burgdorferi Sensu Lato Associated with Cricetid Rodents in Brazil. Microorganisms. 2022;10:204. doi: 10.3390/microorganisms10020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sabitova Y., Rar V., Tikunov A., Yakimenko V., Korallo-Vinarskaya N., Livanova N., Tikunova N. Detection and genetic characterization of a putative novel Borrelia genospecies in Ixodes apronophorus / Ixodes persulcatus / Ixodes trianguliceps sympatric areas in Western Siberia. Ticks Tick-Borne Dis. 2023;14:102075. doi: 10.1016/j.ttbdis.2022.102075. [DOI] [PubMed] [Google Scholar]
- 78.Masuzawa T., Takada N., Kudeken M., Fukui T., Yano Y., Ishiguro F., Kawamura Y., Imai Y., Ezaki T. Borrelia sinica sp. nov., a lyme disease-related Borrelia species isolated in China. Int. J. Syst. Evol. Microbiol. 2001;51:1817–1824. doi: 10.1099/00207713-51-5-1817. [DOI] [PubMed] [Google Scholar]
- 79.Wang G., van Dam A.P., Dankert J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with lyme borreliosis. J. Clin. Microbiol. 1999;37:3025–3028. doi: 10.1128/JCM.37.9.3025-3028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richter D., Schlee D.B., Allgower R., Matuschka F.R. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in Central Europe. Appl. Environ. Microbiol. 2004;70:6414–6419. doi: 10.1128/AEM.70.11.6414-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foldvari G., Farkas R., Lakos A. Borrelia spielmanii erythema migrans, Hungary. Emerg. Infect. Dis. 2005;11:1794–1795. doi: 10.3201/eid1111.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richter D., Postic D., Sertour N., Livey I., Matuschka F.R., Baranton G. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int. J. Syst. Evol. Microbiol. 2006;56:873–881. doi: 10.1099/ijs.0.64050-0. [DOI] [PubMed] [Google Scholar]
- 83.Hiraoka H., Shimada Y., Sakata Y., Watanabe M., Itamoto K., Okuda M., Masuzawa T., Inokuma H. Detection of Borrelia garinii, Borrelia tanukii and Borrelia sp. closely related to Borrelia valaisiana in Ixodes ticks removed from dogs and cats in Japan. Vet. Parasitol. 2007;144:188–192. doi: 10.1016/j.vetpar.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 84.Norte A.C., Araujo P.M., da Silva L.P., Tenreiro P.Q., Ramos J.A., Nuncio M.S., Ze-Ze L., de Carvalho I.L. Characterization Through Multilocus Sequence Analysis of Borrelia turdi Isolates from Portugal. Microb. Ecol. 2016;72:831–839. doi: 10.1007/s00248-015-0660-1. [DOI] [PubMed] [Google Scholar]
- 85.Fukunaga M., Hamase A., Okada K., Nakao M. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from ixodid ticks in Japan: Rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol. Immunol. 1996;40:877–881. doi: 10.1111/j.1348-0421.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 86.Fukunaga M., Hamase A., Okada K., Inoue H., Tsuruta Y., Miyamoto K., Nakao M. Characterization of spirochetes isolated from ticks (Ixodes tanuki, Ixodes turdus, and Ixodes columnae) and comparison of the sequences with those of Borrelia burgdorferi sensu lato strains. Appl. Environ. Microbiol. 1996;62:2338–2344. doi: 10.1128/aem.62.7.2338-2344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palomar A.M., Portillo A., Santibanez P., Mazuelas D., Roncero L., Gutierrez O., Oteo J.A. Presence of Borrelia turdi and Borrelia valaisiana (Spirochaetales: Spirochaetaceae) in Ticks Removed From Birds in the North of Spain, 2009–2011. J. Med. Entomol. 2017;54:243–246. doi: 10.1093/jme/tjw158. [DOI] [PubMed] [Google Scholar]
- 88.Masuzawa T., Pan M.J., Kadosaka T., Kudeken M., Takada N., Yano Y., Imai Y., Yanagihara Y. Characterization and identification of Borrelia isolates as Borrelia valaisiana in Taiwan and Kinmen Islands. Microbiol. Immunol. 2000;44:1003–1009. doi: 10.1111/j.1348-0421.2000.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 89.Hanincova K., Taragelova V., Koci J., Schafer S.M., Hails R., Ullmann A.J., Piesman J., Labuda M., Kurtenbach K. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 2003;69:2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stanek G., Reiter M. The expanding Lyme Borrelia complex—Clinical significance of genomic species? Clin. Microbiol. Infect. 2011;17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 91.Wang G., van Dam A.P., Le Fleche A., Postic D., Peter O., Baranton G., de Boer R., Spanjaard L., Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int. J. Syst. Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 92.Margos G., Sing A., Fingerle V. Published data do not support the notion that Borrelia valaisiana is human pathogenic. Infection. 2017;45:567–569. doi: 10.1007/s15010-017-1032-1. [DOI] [PubMed] [Google Scholar]
- 93.Yun S.M., Lee W.G., Ryou J., Yang S.C., Park S.W., Roh J.Y., Lee Y.J., Park C., Han M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014;20:1358–1361. doi: 10.3201/eid2008.131857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanskul P., Stark H.E., Inlao I. A checklist of ticks of Thailand (Acari: Metastigmata: Ixodoidea) J. Med. Entomol. 1983;20:330–341. doi: 10.1093/jmedent/20.3.330. [DOI] [PubMed] [Google Scholar]
- 95.Lau A.C.C., Qiu Y., Moustafa M.A.M., Nakao R., Shimozuru M., Onuma M., Mohd-Azlan J., Tsubota T. Detection of Borrelia burgdorferi Sensu Lato and Relapsing Fever Borrelia in Feeding Ixodes Ticks and Rodents in Sarawak, Malaysia: New Geographical Records of Borrelia yangtzensis and Borrelia miyamotoi. Pathogens. 2020;9:8460. doi: 10.3390/pathogens9100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Margos G., Chu C.Y., Takano A., Jiang B.G., Liu W., Kurtenbach K., Masuzawa T., Fingerle V., Cao W.C., Kawabata H. Borrelia yangtzensis sp. nov., a rodent-associated species in Asia, is related to Borrelia valaisiana. Int. J. Syst. Evol. Microbiol. 2015;65:3836–3840. doi: 10.1099/ijsem.0.000491. [DOI] [PubMed] [Google Scholar]
- 97.Chu C.Y., Liu W., Jiang B.G., Wang D.M., Jiang W.J., Zhao Q.M., Zhang P.H., Wang Z.X., Tang G.P., Yang H., et al. Novel genospecies of Borrelia burgdorferi sensu lato from rodents and ticks in southwestern China. J. Clin. Microbiol. 2008;46:3130–3133. doi: 10.1128/JCM.01195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trevisan G., Cinco M., Trevisini S., di Meo N., Ruscio M., Forgione P., Bonin S. Borreliae Part 2: Borrelia Relapsing Fever Group and Unclassified Borrelia. Biology. 2021;10:1117. doi: 10.3390/biology10111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Talagrand-Reboul E., Boyer P.H., Bergstrom S., Vial L., Boulanger N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell Infect. Microbiol. 2018;8:98. doi: 10.3389/fcimb.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lopez J., Hovius J.W., Bergstrom S. Pathogenesis of Relapsing Fever. Curr. Issues Mol. Biol. 2021;42:519–550. doi: 10.21775/cimb.042.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zahid H., Alouffi A., Almutairi M.M., Ateeq M., Tanaka T., Chang S.C., Chen C.C., Ali A. Argas persicus and Carios vespertilionis Ticks Infesting Ducks, Domestic Fowls and Bats in Pakistan: First Report on Molecular Survey and Phylogenetic Position of Borrelia anserina. Vet. Sci. 2023;10:628. doi: 10.3390/vetsci10100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cadavid D., Barbour A.G. Neuroborreliosis during relapsing fever: Review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin. Infect. Dis. 1998;26:151–164. doi: 10.1086/516276. [DOI] [PubMed] [Google Scholar]
- 103.Fotso Fotso A., Angelakis E., Mouffok N., Drancourt M., Raoult D. Blood-Borne Candidatus Borrelia algerica in a Patient with Prolonged Fever in Oran, Algeria. Am. J. Trop. Med. Hyg. 2015;93:1070–1073. doi: 10.4269/ajtmh.15-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kandelaki, Maruashvili G.M. On the tick borne relapsing fever. Meditsinskaya Parazitol. Parazit. Bolezn. 1945;14:24–27. [PubMed] [Google Scholar]
- 105.Skerman V.B.D., McGowan V., Sneath P.H.A. Approved Lists of Bacterial Names. Int. J. Syst. Bacteriol. 1980;30:225–420. doi: 10.1099/00207713-30-1-225. [DOI] [Google Scholar]
- 106.Filatov S., Krishnavajhala A., Armstrong B.A., Kneubehl A.R., Nieto N.C., Perez De Leon A.A., Lopez J.E. Isolation and Molecular Characterization of Tick-Borne Relapsing Fever Borrelia Infecting Ornithodoros (Pavlovskyella) verrucosus Ticks Collected in Ukraine. J. Infect. Dis. 2020;221:804–811. doi: 10.1093/infdis/jiz500. [DOI] [PubMed] [Google Scholar]
- 107.Felsenfeld O. Borreliae, Human Relapsing Fever, and Parasite-Vector-Host Relationships. Bacteriol. Rev. 1965;29:46–74. doi: 10.1128/br.29.1.46-74.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leger A. Spirochaete of the Shrew (Crocidura stampflii, Jentink.) Bull. Société Pathol. Exot. 1917;10:280–281. [Google Scholar]
- 109.Novy F.G., Knapp R.E. Studies on Spirillum Obermeieri and Related Organisms. J. Infect. Dis. 1906;3:291–393. doi: 10.1093/infdis/3.3.291. [DOI] [Google Scholar]
- 110.Bergey D.H., Harrison F.C., Breed R.S., Hammer B.W., Huntoon F.M. (Eds) Bergey’s Manual of Determinative Bacteriology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 1925. [Google Scholar]
- 111.Heisch R.B. On a spirochaete isolated from ornithodoros graingeri. Parasitology. 1953;43:133–135. doi: 10.1017/s0031182000018394. [DOI] [PubMed] [Google Scholar]
- 112.Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 113.Garnham P.C. A new blood spirochaete in the grivet monkey; Ceropithecus aethiops. East. Afr. Med. J. 1947;24:47–51. [PubMed] [Google Scholar]
- 114.Davis G.E. The spirochetes. Annu. Rev. Microbiol. 1948;2:305–334. doi: 10.1146/annurev.mi.02.100148.001513. [DOI] [PubMed] [Google Scholar]
- 115.de Buen S. Note préliminaire sur l’épidémiologie de la fièvre récurrente espagnole. Ann. Parasitol. Hum. Comparée. 1926;4:185–192. doi: 10.1051/parasite/1926042185. [DOI] [Google Scholar]
- 116.Fingerle V., Pritsch M., Wachtler M., Margos G., Ruske S., Jung J., Loscher T., Wendtner C., Wieser A. “Candidatus Borrelia kalaharica” Detected from a Febrile Traveller Returning to Germany from Vacation in Southern Africa. PLoS Negl. Trop. Dis. 2016;10:e0004559. doi: 10.1371/journal.pntd.0004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sofiev M. Spirochaeta latyshewi n. sp. of the Recurrens Group. Med. Parasitol. Mosc. 1941;10:267–271. [Google Scholar]
- 118.Hougen K.H. Electron microscopy of Borrelia merionesi and Borrelia recurrentis. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 1974;82:799–809. doi: 10.1111/j.1699-0463.1974.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 119.Rafyi A. Spirochaeta microti n. sp., parasite du campagnol (Microtus sp.) en Iran. Bull. Soc. Pathol. Exot. Fil. 1947;40:149–151. [PubMed] [Google Scholar]
- 120.Naddaf S.R., Ghazinezhad B., Bahramali G., Cutler S.J. Phylogenetic analysis of the spirochete Borrelia microti, a potential agent of relapsing fever in Iran. J. Clin. Microbiol. 2012;50:2873–2876. doi: 10.1128/JCM.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masoumi Asl H., Goya M.M., Vatandoost H., Zahraei S.M., Mafi M., Asmar M., Piazak N., Aghighi Z. The epidemiology of tick-borne relapsing fever in Iran during 1997–2006. Travel Med. Infect. Dis. 2009;7:160–164. doi: 10.1016/j.tmaid.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 122.Dschunkowsky E. Das Rückfallfieber in Persien. Dtsch. Med. Wochenschr. 1913;39:419–420. doi: 10.1055/s-0028-1128212. [DOI] [Google Scholar]
- 123.Zumpt F., Organ D. Strains of spirochaetes isolated from Ornithodoros zumpti Heisch & Guggisberg, and from wild rats in the Cape Province. A preliminary note. S. Afr. J. Lab. Clin. Med. 1961;7:31–35. [PubMed] [Google Scholar]
- 124.Davis G.E. Observations on the biology of the argasid tick, Ornithodoros brasiliensis Aragao, 1923; with the recovery of a spirochete, Borrelia brasiliensis, n. sp. J. Parasitol. 1952;38:473–476. doi: 10.2307/3273927. [DOI] [PubMed] [Google Scholar]
- 125.Lopez J.E., Krishnavahjala A., Garcia M.N., Bermudez S. Tick-Borne Relapsing Fever Spirochetes in the Americas. Vet. Sci. 2016;3:16. doi: 10.3390/vetsci3030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nieto N.C., Teglas M.B., Stewart K.M., Wasley T., Wolff P.L. Detection of relapsing fever spirochetes (Borrelia hermsii and Borrelia coriaceae) in free-ranging mule deer (Odocoileus hemionus) from Nevada, United States. Vector Borne Zoonotic Dis. 2012;12:99–105. doi: 10.1089/vbz.2011.0716. [DOI] [PubMed] [Google Scholar]
- 127.Russell C., Johnson R.C., Burgdorfer W., Lane R.S., Barbour A.G., Hayes S.F., Hyde F.W. Borrelia coriaceae sp. nov.: Putative Agent of Epizootic Bovine Abortion. Int. J. Syst. Bacteriol. 1987;37:72–74. doi: 10.1099/00207713-37-1-72. [DOI] [Google Scholar]
- 128.Nieto N.C., Teglas M.B. Relapsing fever group Borrelia in Southern California rodents. J. Med. Entomol. 2014;51:1029–1034. doi: 10.1603/me14021. [DOI] [PubMed] [Google Scholar]
- 129.Mazzotti L. A New Spirochaete of Relapsing Fever occurring in Mexico. Rev. Inst. Salubr. Enferm. Trop. 1949;10:277–281. [Google Scholar]
- 130.Davis G.E. Species unity or plurality of the relapsing fever spiroehetes. Publ. Amer. Assoc. Adv. Sci. 1942;18:41–47. [Google Scholar]
- 131.Kingry L.C., Anacker M., Pritt B., Bjork J., Respicio-Kingry L., Liu G., Sheldon S., Boxrud D., Strain A., Oatman S., et al. Surveillance for and Discovery of Borrelia Species in US Patients Suspected of Tickborne Illness. Clin. Infect. Dis. 2018;66:1864–1871. doi: 10.1093/cid/cix1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwan T.G., Raffel S.J., Schrumpf M.E., Gill J.S., Piesman J. Characterization of a novel relapsing fever spirochete in the midgut, coxal fluid, and salivary glands of the bat tick Carios kelleyi. Vector Borne Zoonotic Dis. 2009;9:643–647. doi: 10.1089/vbz.2008.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang T., Zhu Y., Zhang Y.Y., Chen J.J., Tian J.B., Xu Q., Jiang B.G., Wang G.L., Golding N., Mehlman M.L., et al. The global distribution and the risk prediction of relapsing fever group Borrelia: A data review with modelling analysis. Lancet Microbe. 2024;5:e442–e451. doi: 10.1016/S2666-5247(23)00396-8. [DOI] [PubMed] [Google Scholar]
- 134.Davis G.E. A relapsing fever spirochete, Borrelia mazzottii (sp. nov.) from Ornithodoros talaje from Mexico. Am. J. Hyg. 1956;63:13–17. doi: 10.1093/oxfordjournals.aje.a119787. [DOI] [PubMed] [Google Scholar]
- 135.Walker R.L., Read D.H., Hayes D.C., Nordhausen R.W. Equine abortion associated with the Borrelia parkeri-B. turicatae tick-borne relapsing fever spirochete group. J. Clin. Microbiol. 2002;40:1558–1562. doi: 10.1128/JCM.40.4.1558-1562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brumpt E. Étude du Spirochaeta turicatae, n. sp. agent de la fièvre récurrente sporadique des Etats-Unis transmis par Ornithodorus turicata. Comptes Rendus Séances Société Biol. 1933;113:1369–1372. [Google Scholar]
- 137.Brumpt E. In: Les Parasites des Invertébrés Hématophages. Lavier A., editor. Volume 207 Vigot Frères; Paris, France: 1921. [Google Scholar]
- 138.Barbour A.G., Maupin G.O., Teltow G.J., Carter C.J., Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 139.Castellaw A.H., Chenney E.F., Varela-Stokes A.S. Tick-borne disease agents in various wildlife from Mississippi. Vector Borne Zoonotic Dis. 2011;11:439–442. doi: 10.1089/vbz.2009.0221. [DOI] [PubMed] [Google Scholar]
- 140.Jordan B.E., Onks K.R., Hamilton S.W., Hayslette S.E., Wright S.M. Detection of Borrelia burgdorferi and Borrelia lonestari in birds in Tennessee. J. Med. Entomol. 2009;46:131–138. doi: 10.1603/033.046.0117. [DOI] [PubMed] [Google Scholar]
- 141.Vazquez Guillamet L.J., Marx G.E., Benjamin W., Pappas P., Lieberman N.A.P., Bachiashvili K., Leal S., Lieberman J.A. Relapsing Fever Caused by Borrelia lonestari after Tick Bite in Alabama, USA. Emerg. Infect. Dis. 2023;29:441–444. doi: 10.3201/eid2902.221281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abdelmaseih R., Ashraf B., Abdelmasih R., Dunn S., Nasser H. Southern Tick-Associated Rash Illness: Florida’s Lyme Disease Variant. Cureus. 2021;13:e15306. doi: 10.7759/cureus.15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fukunaga M., Takahashi Y., Tsuruta Y., Matsushita O., Ralph D., McClelland M., Nakao M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 144.Platonov A.E., Karan L.S., Kolyasnikova N.M., Makhneva N.A., Toporkova M.G., Maleev V.V., Fish D., Krause P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wagemakers A., Jahfari S., de Wever B., Spanjaard L., Starink M.V., de Vries H.J.C., Sprong H., Hovius J.W. Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks Tick-Borne Dis. 2017;8:370–374. doi: 10.1016/j.ttbdis.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 146.Gugliotta J.L., Goethert H.K., Berardi V.P., Telford S.R., 3rd Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin T., Gao L., Seyfang A., Oliver J.H. ‘Candidatus Borrelia texasensis’, from the American dog tick Dermacentor variabilis. Int. J. Syst. Evol. Microbiol. 2005;55:685–693. doi: 10.1099/ijs.0.02864-0. [DOI] [PubMed] [Google Scholar]
- 148.Laveran A. Sur la spirillose des bovidés. CR Acad. Sci. Paris. 1903;136:939–941. [Google Scholar]
- 149.Theiler A. Transmission and inoculability of Spirillum Theileri (Laveran) Proc. R. Soc. Lond. B. 1905;76:504–506. doi: 10.1098/rspb.1905.0043. [DOI] [Google Scholar]
- 150.Sakharoff M.N. Spirochaeta anserina et la septicémie des oies. Ann. Inst. Pasteur. 1891;5:564–566. [Google Scholar]
- 151.Fabbi M., Sambri V., Marangoni A., Magnino S., Solari Basano F., Cevenini R., Genchi C. Borrelia in pigeons: No serological evidence of Borrelia burgdorferi infection. Zentralbl. Vet. B. 1995;42:503–507. doi: 10.1111/j.1439-0450.1995.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 152.Hovind-Hougen K. A morphological characterization of Borrelia anserina. Pt 1Microbiology (Reading) 1995;141:79–83. doi: 10.1099/00221287-141-1-79. [DOI] [PubMed] [Google Scholar]
- 153.Horefti E. The Importance of the One Health Concept in Combating Zoonoses. Pathogens. 2023;12:977. doi: 10.3390/pathogens12080977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dye C. One Health as a catalyst for sustainable development. Nat. Microbiol. 2022;7:467–468. doi: 10.1038/s41564-022-01076-1. [DOI] [PubMed] [Google Scholar]
- 155.One Health High-Level Expert P., Adisasmito W.B., Almuhairi S., Behravesh C.B., Bilivogui P., Bukachi S.A., Casas N., Cediel Becerra N., Charron D.F., Chaudhary A., et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022;18:e1010537. doi: 10.1371/journal.ppat.1010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Radolf J.D., Strle K., Lemieux J.E., Strle F. Lyme Disease in Humans. Curr. Issues Mol. Biol. 2021;42:333–384. doi: 10.21775/cimb.042.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stanek G., Strle F. Lyme borreliosis. Lancet. 2003;362:1639–1647. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- 158.Steere A.C. Lyme disease. N. Engl. J. Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 159.Kullberg B.J., Vrijmoeth H.D., van de Schoor F., Hovius J.W. Lyme borreliosis: Diagnosis and management. BMJ. 2020;369:m1041. doi: 10.1136/bmj.m1041. [DOI] [PubMed] [Google Scholar]
- 160.Huppertz H.I., Bohme M., Standaert S.M., Karch H., Plotkin S.A. Incidence of Lyme borreliosis in the Wurzburg region of Germany. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 1999;18:697–703. doi: 10.1007/s100960050381. [DOI] [PubMed] [Google Scholar]
- 161.Steere A.C., Sikand V.K. The presenting manifestations of Lyme disease and the outcomes of treatment. N. Engl. J. Med. 2003;348:2472–2474. doi: 10.1056/NEJM200306123482423. [DOI] [PubMed] [Google Scholar]
- 162.Sanchez J.L. Clinical Manifestations and Treatment of Lyme Disease. Clin. Lab. Med. 2015;35:765–778. doi: 10.1016/j.cll.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 163.Nadelman R.B. Erythema migrans. Infect. Dis. Clin. N. Am. 2015;29:211–239. doi: 10.1016/j.idc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 164.Smith R.P., Schoen R.T., Rahn D.W., Sikand V.K., Nowakowski J., Parenti D.L., Holman M.S., Persing D.H., Steere A.C. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann. Intern. Med. 2002;136:421–428. doi: 10.7326/0003-4819-136-6-200203190-00005. [DOI] [PubMed] [Google Scholar]
- 165.Wormser G.P., McKenna D., Carlin J., Nadelman R.B., Cavaliere L.F., Holmgren D., Byrne D.W., Nowakowski J. Brief communication: Hematogenous dissemination in early Lyme disease. Ann. Intern. Med. 2005;142:751–755. doi: 10.7326/0003-4819-142-9-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 166.Nadelman R.B., Nowakowski J., Forseter G., Goldberg N.S., Bittker S., Cooper D., Aguero-Rosenfeld M., Wormser G.P. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am. J. Med. 1996;100:502–508. doi: 10.1016/s0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 167.Steere A.C., Bartenhagen N.H., Craft J.E., Hutchinson G.J., Newman J.H., Rahn D.W., Sigal L.H., Spieler P.N., Stenn K.S., Malawista S.E. The early clinical manifestations of Lyme disease. Ann. Intern. Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 168.Wormser G.P., Dattwyler R.J., Shapiro E.D., Halperin J.J., Steere A.C., Klempner M.S., Krause P.J., Bakken J.S., Strle F., Stanek G., et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 169.Koedel U., Fingerle V., Pfister H.W. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol. 2015;11:446–456. doi: 10.1038/nrneurol.2015.121. [DOI] [PubMed] [Google Scholar]
- 170.Knudtzen F.C., Eikeland R., Bremell D., Quist-Paulsen E., Johansen I.S., Solheim A.M., Skarphedinsson S. Lyme neuroborreliosis with encephalitis; a systematic literature review and a Scandinavian cohort study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022;28:649–656. doi: 10.1016/j.cmi.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 171.Lochhead R.B., Strle K., Arvikar S.L., Weis J.J., Steere A.C. Lyme arthritis: Linking infection, inflammation and autoimmunity. Nat. Rev. Rheumatol. 2021;17:449–461. doi: 10.1038/s41584-021-00648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Forrester J.D., Meiman J., Mullins J., Nelson R., Ertel S.H., Cartter M., Brown C.M., Lijewski V., Schiffman E., Neitzel D., et al. Notes from the field: Update on Lyme carditis, groups at high risk, and frequency of associated sudden cardiac death—United States. MMWR Morb. Mortal. Wkly. Rep. 2014;63:982–983. [PMC free article] [PubMed] [Google Scholar]
- 173.Stanek G., Klein J., Bittner R., Glogar D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N. Engl. J. Med. 1990;322:249–252. doi: 10.1056/NEJM199001253220407. [DOI] [PubMed] [Google Scholar]
- 174.Bobe J.R., Jutras B.L., Horn E.J., Embers M.E., Bailey A., Moritz R.L., Zhang Y., Soloski M.J., Ostfeld R.S., Marconi R.T., et al. Recent Progress in Lyme Disease and Remaining Challenges. Front. Med. 2021;8:666554. doi: 10.3389/fmed.2021.666554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Motamed M., Liblik K., Miranda-Arboleda A.F., Wamboldt R., Wang C.N., Cingolani O., Rebman A.W., Novak C.B., Aucott J.N., Farina J.M., et al. Disseminated Lyme disease and dilated cardiomyopathy: A systematic review. Trends Cardiovasc. Med. 2023;33:531–536. doi: 10.1016/j.tcm.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 176.Hubalek Z. Epidemiology of lyme borreliosis. Curr. Probl. Dermatol. 2009;37:31–50. doi: 10.1159/000213069. [DOI] [PubMed] [Google Scholar]
- 177.Sykes R.A., Makiello P. An estimate of Lyme borreliosis incidence in Western Europedagger. J. Public Health. 2017;39:74–81. doi: 10.1093/pubmed/fdw017. [DOI] [PubMed] [Google Scholar]
- 178.Ai C.X., Zhang W.F., Zhao J.H. Sero-epidemiology of Lyme disease in an endemic area in China. Microbiol. Immunol. 1994;38:505–509. doi: 10.1111/j.1348-0421.1994.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 179.von Fricken M.E., Rolomjav L., Illar M., Altantogtokh D., Hogan K.M., Uyanga B., Ganbold D., Tsogbadrakh N. Geographic Range of Lyme Borreliosis in Mongolia. Vector Borne Zoonotic Dis. 2019;19:658–661. doi: 10.1089/vbz.2018.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schwartz A.M., Hinckley A.F., Mead P.S., Hook S.A., Kugeler K.J. Surveillance for Lyme Disease—United States, 2008–2015. MMWR Surveill. Summ. 2017;66:1–12. doi: 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kugeler K.J., Farley G.M., Forrester J.D., Mead P.S. Geographic Distribution and Expansion of Human Lyme Disease, United States. Emerg. Infect. Dis. 2015;21:1455–1457. doi: 10.3201/eid2108.141878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gasmi S., Ogden N.H., Ripoche M., Leighton P.A., Lindsay R.L., Nelder M.P., Rees E., Bouchard C., Vrbova L., Rusk R., et al. Detection of municipalities at-risk of Lyme disease using passive surveillance of Ixodes scapularis as an early signal: A province-specific indicator in Canada. PLoS ONE. 2019;14:e0212637. doi: 10.1371/journal.pone.0212637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-Pena A., George J.C., Golovljova I., Jaenson T.G., Jensen J.K., Jensen P.M., et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1–11. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Smith R., Takkinen J. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Wkly. Releases. 2006;11:2977. doi: 10.2807/esw.11.25.02977-en. [DOI] [PubMed] [Google Scholar]
- 185.Semenza J.C., Suk J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018;365:fnx244. doi: 10.1093/femsle/fnx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Piesman J., Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129:S191–S220. doi: 10.1017/s0031182003004694. [DOI] [PubMed] [Google Scholar]
- 187.Maggi R.G., Reichelt S., Toliver M., Engber B. Borrelia species in Ixodes affinis and Ixodes scapularis ticks collected from the coastal plain of North Carolina. Ticks Tick-Borne Dis. 2010;1:168–171. doi: 10.1016/j.ttbdis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 188.Stark J.H., Li X., Zhang J.C., Burn L., Valluri S.R., Liang J., Pan K., Fletcher M.A., Simon R., Jodar L., et al. Systematic Review and Meta-analysis of Lyme Disease Data and Seropositivity for Borrelia burgdorferi, China, 2005–2020. Emerg. Infect. Dis. 2022;28:2389–2397. doi: 10.3201/eid2812.212612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eisen L., Eisen R.J. Critical Evaluation of the Linkage Between Tick-Based Risk Measures and the Occurrence of Lyme Disease Cases. J. Med. Entomol. 2016;53:1050–1062. doi: 10.1093/jme/tjw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Piesman J., Schneider B.S., Zeidner N.S. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J. Clin. Microbiol. 2001;39:4145–4148. doi: 10.1128/JCM.39.11.4145-4148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.des Vignes F., Piesman J., Heffernan R., Schulze T.L., Stafford K.C., 3rd, Fish D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis. 2001;183:773–778. doi: 10.1086/318818. [DOI] [PubMed] [Google Scholar]
- 192.Mannelli A., Bertolotti L., Gern L., Gray J. Ecology of Borrelia burgdorferi sensu lato in Europe: Transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol. Rev. 2012;36:837–861. doi: 10.1111/j.1574-6976.2011.00312.x. [DOI] [PubMed] [Google Scholar]
- 193.Stanek G., Strle F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018;42:233–258. doi: 10.1093/femsre/fux047. [DOI] [PubMed] [Google Scholar]
- 194.Rollend L., Fish D., Childs J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick-Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 195.Markowitz L.E., Steere A.C., Benach J.L., Slade J.D., Broome C.V. Lyme disease during pregnancy. JAMA. 1986;255:3394–3396. doi: 10.1001/jama.1986.03370240064038. [DOI] [PubMed] [Google Scholar]
- 196.Diaz P., Arnal J.L., Remesar S., Perez-Creo A., Venzal J.M., Vazquez-Lopez M.E., Prieto A., Fernandez G., Lopez C.M., Panadero R., et al. Molecular identification of Borrelia spirochetes in questing Ixodes ricinus from northwestern Spain. Parasit Vectors. 2017;10:615. doi: 10.1186/s13071-017-2574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Remesar S., Matute R., Diaz P., Martinez-Calabuig N., Prieto A., Diaz-Cao J.M., Lopez-Lorenzo G., Fernandez G., Lopez C., Panadero R., et al. Tick-borne pathogens in ticks from urban and suburban areas of north-western Spain: Importance of Ixodes frontalis harbouring zoonotic pathogens. Med. Vet. Entomol. 2023;37:499–510. doi: 10.1111/mve.12648. [DOI] [PubMed] [Google Scholar]
- 198.Diaz-Cao J.M., Adaszek L., Dziegiel B., Paniagua J., Caballero-Gomez J., Winiarczyk S., Winiarczyk D., Cano-Terriza D., Garcia-Bocanegra I. Prevalence of selected tick-borne pathogens in wild ungulates and ticks in southern Spain. Transbound. Emerg. Dis. 2022;69:1084–1094. doi: 10.1111/tbed.14065. [DOI] [PubMed] [Google Scholar]
- 199.Espi A., Del Cerro A., Somoano A., Garcia V., Prieto J.M., Barandika J.F., Garcia-Perez A.L. Borrelia burgdorferi sensu lato prevalence and diversity in ticks and small mammals in a Lyme borreliosis endemic Nature Reserve in North-Western Spain. Incidence in surrounding human populations. Enferm. Infect. Microbiol. Clin. 2017;35:563–568. doi: 10.1016/j.eimc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 200.Del Cerro A., Oleaga A., Somoano A., Barandika J.F., Garcia-Perez A.L., Espi A. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks Tick-Borne Dis. 2022;13:101961. doi: 10.1016/j.ttbdis.2022.101961. [DOI] [PubMed] [Google Scholar]
- 201.Lledo L., Gegundez M.I., Gimenez-Pardo C., Alamo R., Fernandez-Soto P., Nuncio M.S., Saz J.V. A seventeen-year epidemiological surveillance study of Borrelia burgdorferi infections in two provinces of northern Spain. Int. J. Environ. Res. Public Health. 2014;11:1661–1672. doi: 10.3390/ijerph110201661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Marchant A., Le Coupanec A., Joly C., Perthame E., Sertour N., Garnier M., Godard V., Ferquel E., Choumet V. Infection of Ixodes ricinus by Borrelia burgdorferi sensu lato in peri-urban forests of France. PLoS ONE. 2017;12:e0183543. doi: 10.1371/journal.pone.0183543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cotte V., Bonnet S., Cote M., Vayssier-Taussat M. Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis. 2010;10:723–730. doi: 10.1089/vbz.2009.0066. [DOI] [PubMed] [Google Scholar]
- 204.Aubry C., Socolovschi C., Raoult D., Parola P. Bacterial agents in 248 ticks removed from people from 2002 to 2013. Ticks Tick-Borne Dis. 2016;7:475–481. doi: 10.1016/j.ttbdis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 205.Rataud A., Galon C., Bournez L., Henry P.Y., Marsot M., Moutailler S. Diversity of Tick-Borne Pathogens in Tick Larvae Feeding on Breeding Birds in France. Pathogens. 2022;11:946. doi: 10.3390/pathogens11080946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cicculli V., Capai L., Quilichini Y., Masse S., Fernandez-Alvarez A., Minodier L., Bompard P., Charrel R., Falchi A. Molecular investigation of tick-borne pathogens in ixodid ticks infesting domestic animals (cattle and sheep) and small rodents (black rats) of Corsica, France. Ticks Tick-Borne Dis. 2019;10:606–613. doi: 10.1016/j.ttbdis.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 207.Bonnet S., de la Fuente J., Nicollet P., Liu X., Madani N., Blanchard B., Maingourd C., Alongi A., Torina A., Fernandez de Mera I.G., et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis. 2013;13:226–236. doi: 10.1089/vbz.2011.0933. [DOI] [PubMed] [Google Scholar]
- 208.Pistone D., Pajoro M., Novakova E., Vicari N., Gaiardelli C., Vigano R., Luzzago C., Montagna M., Lanfranchi P. Ticks and bacterial tick-borne pathogens in Piemonte region, Northwest Italy. Exp. Appl. Acarol. 2017;73:477–491. doi: 10.1007/s10493-017-0202-2. [DOI] [PubMed] [Google Scholar]
- 209.Ragagli C., Mannelli A., Ambrogi C., Bisanzio D., Ceballos L.A., Grego E., Martello E., Selmi M., Tomassone L. Presence of host-seeking Ixodes ricinus and their infection with Borrelia burgdorferi sensu lato in the Northern Apennines, Italy. Exp. Appl. Acarol. 2016;69:167–178. doi: 10.1007/s10493-016-0030-9. [DOI] [PubMed] [Google Scholar]
- 210.Audino T., Pautasso A., Bellavia V., Carta V., Ferrari A., Verna F., Grattarola C., Iulini B., Pintore M.D., Bardelli M., et al. Ticks infesting humans and associated pathogens: A cross-sectional study in a 3-year period (2017–2019) in northwest Italy. Parasit Vectors. 2021;14:136. doi: 10.1186/s13071-021-04603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tomassone L., Grego E., Auricchio D., Iori A., Giannini F., Rambozzi L. Lyme borreliosis spirochetes and spotted fever group rickettsiae in ixodid ticks from Pianosa island, Tuscany Archipelago, Italy. Vector Borne Zoonotic Dis. 2013;13:84–91. doi: 10.1089/vbz.2012.1046. [DOI] [PubMed] [Google Scholar]
- 212.Rollins R.E., Schaper S., Kahlhofer C., Frangoulidis D., Strauss A.F.T., Cardinale M., Springer A., Strube C., Bakkes D.K., Becker N.S., et al. Ticks (Acari: Ixodidae) on birds migrating to the island of Ponza, Italy, and the tick-borne pathogens they carry. Ticks Tick-Borne Dis. 2021;12:101590. doi: 10.1016/j.ttbdis.2020.101590. [DOI] [PubMed] [Google Scholar]
- 213.Susnjar J., Cerar Kisek T., Strasek Smrdel K., Ruzic-Sabljic E., Adam K., Ivovic V. Detection, identification and genotyping of Borrelia spp. in ticks of Coastal-Karst and Littoral-Inner Carniola regions in Slovenia. Folia Parasitol. 2023;70:007. doi: 10.14411/fp.2023.007. [DOI] [PubMed] [Google Scholar]
- 214.Norte A.C., Margos G., Becker N.S., Albino Ramos J., Nuncio M.S., Fingerle V., Araujo P.M., Adamik P., Alivizatos H., Barba E., et al. Host dispersal shapes the population structure of a tick-borne bacterial pathogen. Mol. Ecol. 2020;29:485–501. doi: 10.1111/mec.15336. [DOI] [PubMed] [Google Scholar]
- 215.Rijpkema S., Golubic D., Molkenboer M., Verbeek-De Kruif N., Schellekens J. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 1996;20:23–30. doi: 10.1007/BF00051474. [DOI] [PubMed] [Google Scholar]
- 216.Norte A.C., Boyer P.H., Castillo-Ramirez S., Chvostac M., Brahami M.O., Rollins R.E., Woudenberg T., Didyk Y.M., Derdakova M., Nuncio M.S., et al. The Population Structure of Borrelia lusitaniae Is Reflected by a Population Division of Its Ixodes Vector. Microorganisms. 2021;9:933. doi: 10.3390/microorganisms9050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Guner E.S., Hashimoto N., Takada N., Kaneda K., Imai Y., Masuzawa T. First isolation and characterization of Borrelia burgdorferi sensu lato strains from Ixodes ricinus ticks in Turkey. J. Med. Microbiol. 2003;52:807–813. doi: 10.1099/jmm.0.05205-0. [DOI] [PubMed] [Google Scholar]
- 218.Orkun O., Cakmak A. Molecular identification of tick-borne bacteria in wild animals and their ticks in Central Anatolia, Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2019;63:58–65. doi: 10.1016/j.cimid.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 219.Orkun O., Karaer Z., Cakmak A., Nalbantoglu S. Identification of tick-borne pathogens in ticks feeding on humans in Turkey. PLoS Negl. Trop. Dis. 2014;8:e3067. doi: 10.1371/journal.pntd.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Polat E., Altinkum S.M., Bagdatli Y., Baykara O. The tick fauna in Istanbul, Turkey, from 2013 to 2017 and identification of their pathogens by multiplex PCR: An epidemiological study. Exp. Appl. Acarol. 2021;84:825–834. doi: 10.1007/s10493-021-00642-2. [DOI] [PubMed] [Google Scholar]
- 221.Ashour R., Hamza D., Kadry M., Sabry M.A. The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt. Vet. Sci. 2023;10:141. doi: 10.3390/vetsci10020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Elhelw R.A., El-Enbaawy M.I., Samir A. Lyme borreliosis: A neglected zoonosis in Egypt. Acta Trop. 2014;140:188–192. doi: 10.1016/j.actatropica.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 223.Younsi H., Postic D., Baranton G., Bouattour A. High prevalence of Borrelia lusitaniae in Ixodes ricinus ticks in Tunisia. Eur. J. Epidemiol. 2001;17:53–56. doi: 10.1023/a:1010928731281. [DOI] [PubMed] [Google Scholar]
- 224.Younsi H., Sarih M., Jouda F., Godfroid E., Gern L., Bouattour A., Baranton G., Postic D. Characterization of Borrelia lusitaniae isolates collected in Tunisia and Morocco. J. Clin. Microbiol. 2005;43:1587–1593. doi: 10.1128/JCM.43.4.1587-1593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Benredjem W., Leulmi H., Bitam I., Raoult D., Parola P. Borrelia garinii and Rickettsia monacensis in Ixodes ricinus ticks, Algeria. Emerg. Infect. Dis. 2014;20:1776–1777. doi: 10.3201/eid2010.140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sarih M., Jouda F., Gern L., Postic D. First isolation of Borrelia burgdorferi sensu lato from Ixodes ricinus ticks in Morocco. Vector Borne Zoonotic Dis. 2003;3:133–139. doi: 10.1089/153036603768395834. [DOI] [PubMed] [Google Scholar]
- 227.LoGiudice K., Ostfeld R.S., Schmidt K.A., Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gern L., Estrada-Pena A., Frandsen F., Gray J.S., Jaenson T.G., Jongejan F., Kahl O., Korenberg E., Mehl R., Nuttall P.A. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralbl Bakteriol. 1998;287:196–204. doi: 10.1016/s0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- 229.Margos G., Vollmer S.A., Ogden N.H., Fish D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect. Genet. Evol. 2011;11:1545–1563. doi: 10.1016/j.meegid.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Craine N.G., Nuttall P.A., Marriott A.C., Randolph S.E. Role of grey squirrels and pheasants in the transmission of Borrelia burgdorferi sensu lato, the Lyme disease spirochaete, in the U.K. Folia Parasitol. 1997;44:155–160. [PubMed] [Google Scholar]
- 231.Slajchert T., Kitron U.D., Jones C.J., Mannelli A. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern Illinois, USA. J. Wildl. Dis. 1997;33:40–46. doi: 10.7589/0090-3558-33.1.40. [DOI] [PubMed] [Google Scholar]
- 232.Donahue J.G., Piesman J., Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 233.Saint Girons I., Gern L., Gray J.S., Guy E.C., Korenberg E., Nuttall P.A., Rijpkema S.G., Schonberg A., Stanek G., Postic D. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentralbl Bakteriol. 1998;287:190–195. doi: 10.1016/s0934-8840(98)80120-5. [DOI] [PubMed] [Google Scholar]
- 234.Skarphedinsson S., Jensen P.M., Kristiansen K. Survey of tickborne infections in Denmark. Emerg. Infect. Dis. 2005;11:1055–1061. doi: 10.3201/eid1107.041265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jaenson T.G., Talleklint L. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J. Med. Entomol. 1992;29:813–817. doi: 10.1093/jmedent/29.5.813. [DOI] [PubMed] [Google Scholar]
- 236.Roome A., Hill L., Al-Feghali V., Murnock C.G., Goodsell J.A., Spathis R., Garruto R.M. Impact of white-tailed deer on the spread of Borrelia burgdorferi. Med. Vet. Entomol. 2017;31:1–5. doi: 10.1111/mve.12191. [DOI] [PubMed] [Google Scholar]
- 237.Rand P.W., Lubelczyk C., Lavigne G.R., Elias S., Holman M.S., Lacombe E.H., Smith R.P., Jr. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 2003;40:179–184. doi: 10.1603/0022-2585-40.2.179. [DOI] [PubMed] [Google Scholar]
- 238.Delgado S., Carmenes P. Seroepidemiological survey for Borrelia burgdorferi (Lyme disease) in dogs from northwestern of Spain. Eur. J. Epidemiol. 1995;11:321–324. doi: 10.1007/BF01719437. [DOI] [PubMed] [Google Scholar]
- 239.Lledo L., Serrano J.L., Isabel Gegundez M., Gimenez-Pardo C., Saz J.V. Antibodies to Rickettsia spp. and Borrelia burgdorferi in Spanish Wild Red Foxes (Vulpes vulpes) J. Wildl. Dis. 2016;52:122–125. doi: 10.7589/2015-03-074. [DOI] [PubMed] [Google Scholar]
- 240.Sobrino R., Gortazar C. Seroprevalence of antibodies to Borrelia burgdorferi in wild canids in Spain. Vet. Rec. 2008;162:248–249. doi: 10.1136/vr.162.8.248. [DOI] [PubMed] [Google Scholar]
- 241.Ortuno A., Castella J., Marco I., Ruiz M., Lavin S. Prevalence of antibodies to Borrelia burgdorferi sensu lato in southern chamois (Rupicapra pyrenaica) in Spain. J. Vet. Med. B Infect. Dis. Vet. Public. Health. 2003;50:253–254. doi: 10.1046/j.1439-0450.2003.00661.x. [DOI] [PubMed] [Google Scholar]
- 242.Marsot M., Chapuis J.L., Gasqui P., Dozieres A., Masseglia S., Pisanu B., Ferquel E., Vourc’h G. Introduced Siberian chipmunks (Tamias sibiricus barberi) contribute more to lyme borreliosis risk than native reservoir rodents. PLoS ONE. 2013;8:e55377. doi: 10.1371/journal.pone.0055377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vourc’h G., Marmet J., Chassagne M., Bord S., Chapuis J.L. Borrelia burgdorferi Sensu Lato in Siberian chipmunks (Tamias sibiricus) introduced in suburban forests in France. Vector Borne Zoonotic Dis. 2007;7:637–641. doi: 10.1089/vbz.2007.0111. [DOI] [PubMed] [Google Scholar]
- 244.Marsot M., Sigaud M., Chapuis J.L., Ferquel E., Cornet M., Vourc’h G. Introduced Siberian chipmunks (Tamias sibiricus barberi) harbor more-diverse Borrelia burgdorferi sensu lato genospecies than native bank voles (Myodes glareolus) Appl. Environ. Microbiol. 2011;77:5716–5721. doi: 10.1128/AEM.01846-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jacquot M., Bisseux M., Abrial D., Marsot M., Ferquel E., Chapuis J.L., Vourc’h G., Bailly X. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. PLoS ONE. 2014;9:e88581. doi: 10.1371/journal.pone.0088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pisanu B., Chapuis J.L., Dozieres A., Basset F., Poux V., Vourc’h G. High prevalence of Borrelia burgdorferi s.l. in the European red squirrel Sciurus vulgaris in France. Ticks Tick-Borne Dis. 2014;5:1–6. doi: 10.1016/j.ttbdis.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 247.Pantchev N., Schaper R., Limousin S., Norden N., Weise M., Lorentzen L. Occurrence of Dirofilaria immitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in domestic dogs in France: Results of a countrywide serologic survey. Parasitol. Res. 2009;105((Suppl. 1)):S101–S114. doi: 10.1007/s00436-009-1501-2. [DOI] [PubMed] [Google Scholar]
- 248.Maurizi L., Marie J.L., Aoun O., Courtin C., Gorsane S., Chal D., Davoust B. Seroprevalence survey of equine Lyme borreliosis in France and in sub-Saharan Africa. Vector Borne Zoonotic Dis. 2010;10:535–537. doi: 10.1089/vbz.2009.0083. [DOI] [PubMed] [Google Scholar]
- 249.Pichon B., Gilot B., Perez-Eid C. Detection of spirochaetes of Borrelia burgdorferi complexe in the skin of cervids by PCR and culture. Eur. J. Epidemiol. 2000;16:869–873. doi: 10.1023/a:1007646216035. [DOI] [PubMed] [Google Scholar]
- 250.Pascucci I., Di Domenico M., Dall’Acqua F., Sozio G., Camma C. Detection of Lyme Disease and Q Fever Agents in Wild Rodents in Central Italy. Vector Borne Zoonotic Dis. 2015;15:404–411. doi: 10.1089/vbz.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Martello E., Mannelli A., Grego E., Ceballos L.A., Ragagli C., Stella M.C., Tomassone L. Borrelia burgdorferi sensu lato and spotted fever group rickettsiae in small rodents and attached ticks in the Northern Apennines, Italy. Ticks Tick-Borne Dis. 2019;10:862–867. doi: 10.1016/j.ttbdis.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 252.Sgroi G., Iatta R., Veneziano V., Bezerra-Santos M.A., Lesiczka P., Hrazdilova K., Annoscia G., D’Alessio N., Golovchenko M., Rudenko N., et al. Molecular survey on tick-borne pathogens and Leishmania infantum in red foxes (Vulpes vulpes) from southern Italy. Ticks Tick-Borne Dis. 2021;12:101669. doi: 10.1016/j.ttbdis.2021.101669. [DOI] [PubMed] [Google Scholar]
- 253.Ebani V.V., Bertelloni F., Torracca B., Cerri D. Serological survey of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann. Agric. Environ. Med. 2014;21:671–675. doi: 10.5604/12321966.1129912. [DOI] [PubMed] [Google Scholar]
- 254.Piantedosi D., Neola B., D’Alessio N., Di Prisco F., Santoro M., Pacifico L., Sgroi G., Auletta L., Buch J., Chandrashekar R., et al. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and D. immitis in hunting dogs from southern Italy. Parasitol. Res. 2017;116:2651–2660. doi: 10.1007/s00436-017-5574-z. [DOI] [PubMed] [Google Scholar]
- 255.Ebani V.V., Bertelloni F., Pinzauti P., Cerri D. Seroprevalence of Leptospira spp. and Borrelia burgdorferi sensu lato in Italian horses. Ann. Agric. Environ. Med. 2012;19:237–240. [PubMed] [Google Scholar]
- 256.Veronesi F., Laus F., Passamonti F., Tesei B., Piergili Fioretti D., Genchi C. Occurrence of Borrelia lusitaniae infection in horses. Vet. Microbiol. 2012;160:535–538. doi: 10.1016/j.vetmic.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 257.Mendoza-Roldan J.A., Colella V., Lia R.P., Nguyen V.L., Barros-Battesti D.M., Iatta R., Dantas-Torres F., Otranto D. Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasit Vectors. 2019;12:35. doi: 10.1186/s13071-019-3286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ebani V.V., Poli A., Rocchigiani G., Bertelloni F., Nardoni S., Papini R.A., Mancianti F. Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy. Asian Pac. J. Trop. Med. 2016;9:465–469. doi: 10.1016/j.apjtm.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 259.Grassi L., Drigo M., Zelena H., Pasotto D., Cassini R., Mondin A., Franzo G., Tucciarone C.M., Ossola M., Vidorin E., et al. Wild ungulates as sentinels of flaviviruses and tick-borne zoonotic pathogen circulation: An Italian perspective. BMC Vet. Res. 2023;19:155. doi: 10.1186/s12917-023-03717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ebani V.V., Bertelloni F., Mani P. Serological evidence of exposure to zoonotic tick-borne bacteria in pheasants (Phasianus colchicus) Ann. Agric. Environ. Med. 2017;24:82–85. doi: 10.5604/12321966.1234004. [DOI] [PubMed] [Google Scholar]
- 261.Ebani V.V., Bertelloni F., Mani P. Molecular survey on zoonotic tick-borne bacteria and chlamydiae in feral pigeons (Columba livia domestica) Asian Pac. J. Trop. Med. 2016;9:324–327. doi: 10.1016/j.apjtm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 262.Ebani V.V., Rocchigiani G., Bertelloni F., Nardoni S., Leoni A., Nicoloso S., Mancianti F. Molecular survey on the presence of zoonotic arthropod-borne pathogens in wild red deer (Cervus elaphus) Comp. Immunol. Microbiol. Infect. Dis. 2016;47:77–80. doi: 10.1016/j.cimid.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 263.Cuteri V., Diverio S., Carnieletto P., Turilli C., Valente C. Serological survey for antibodies against selected infectious agents among fallow deer (Dama dama) in central Italy. Zentralbl. Vet. B. 1999;46:545–549. doi: 10.1111/j.1439-0450.1999.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 264.Cerar T., Korva M., Avsic-Zupanc T., Ruzic-Sabljic E. Detection, identification and genotyping of Borrellia spp. in rodents in Slovenia by PCR and culture. BMC Vet. Res. 2015;11:188. doi: 10.1186/s12917-015-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Strle F. Lyme borreliosis in Slovenia. Zentralbl Bakteriol. 1999;289:643–652. doi: 10.1016/s0934-8840(99)80023-1. [DOI] [PubMed] [Google Scholar]
- 266.Miro G., Wright I., Michael H., Burton W., Hegarty E., Rodon J., Buch J., Pantchev N., von Samson-Himmelstjerna G. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasit Vectors. 2022;15:189. doi: 10.1186/s13071-022-05316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tadin A., Tokarz R., Markotic A., Margaletic J., Turk N., Habus J., Svoboda P., Vucelja M., Desai A., Jain K., et al. Molecular Survey of Zoonotic Agents in Rodents and Other Small Mammals in Croatia. Am. J. Trop. Med. Hyg. 2016;94:466–473. doi: 10.4269/ajtmh.15-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mrljak V., Kules J., Mihaljevic Z., Torti M., Gotic J., Crnogaj M., Zivicnjak T., Mayer I., Smit I., Bhide M., et al. Prevalence and Geographic Distribution of Vector-Borne Pathogens in Apparently Healthy Dogs in Croatia. Vector Borne Zoonotic Dis. 2017;17:398–408. doi: 10.1089/vbz.2016.1990. [DOI] [PubMed] [Google Scholar]
- 269.Jurkovic D., Beck A., Huber D., Mihaljevic Z., Polkinghorne A., Martinkovic F., Lukacevic D., Pilat M., Brezak R., Bosnic S., et al. Seroprevalence of vector-borne pathogens in dogs from Croatia. Parasitol. Res. 2019;118:347–352. doi: 10.1007/s00436-018-6129-7. [DOI] [PubMed] [Google Scholar]
- 270.Myrseli T., Schönberg A., Simaku A., Çomo N., Pipero P., Bërxholi K., Bino S., Kraja D. The current situation of Lyme borreliosis and the first seroepidemiology survey in dogs in Albania. Albanian Med. J. 2018;2:19–23. [Google Scholar]
- 271.Angelou A., Gelasakis A.I., Verde N., Pantchev N., Schaper R., Chandrashekar R., Papadopoulos E. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasit Vectors. 2019;12:283. doi: 10.1186/s13071-019-3543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Athanasiou L.V., Kontos V.I., Kritsepi Konstantinou M., Polizopoulou Z.S., Rousou X.A., Christodoulopoulos G. Cross-Sectional Serosurvey and Factors Associated with Exposure of Dogs to Vector-Borne Pathogens in Greece. Vector Borne Zoonotic Dis. 2019;19:923–928. doi: 10.1089/vbz.2019.2471. [DOI] [PubMed] [Google Scholar]
- 273.Athanasiou L.V., Spanou V.M., Katsogiannou E.G., Katsoulos P.D. Hematological Features in Sheep with IgG and IgM Antibodies against Borrelia burgdorferi sensu lato. Pathogens. 2021;10:164. doi: 10.3390/pathogens10020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Athanasiou L.V., Katsogiannou E.G., Tyrnenopoulou P., Gougoulis D., Apostolidis K.N., Papadakis S.M., Kokkinaki K.C.G., Papatsiros V.G., Tsokana C.N. Evidence of Horse Exposure to Anaplasma phagocytophilum, Borrelia burgdorferi, and Leishmania infantum in Greece through the Detection of IgG Antibodies in Serum and in an Alternative Diagnostic Sample-The Saliva. Biomolecules. 2023;13:1374. doi: 10.3390/biom13091374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bhide M., Yilmaz Z., Golcu E., Torun S., Mikula I. Seroprevalence of anti-Borrelia burgdorferi antibodies in dogs and horses in Turkey. Ann. Agric. Environ. Med. 2008;15:85–90. [PubMed] [Google Scholar]
- 276.Guner E.S., Watanabe M., Kadosaka T., Polat E., Gargili A., Gulanber A., Ohashi N., Kaneda K., Imai Y., Masuzawa T. Seroepidemiology of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in wild mice captured in northern Turkey. Epidemiol. Infect. 2005;133:331–336. doi: 10.1017/s0950268804003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Muz M.N., Erat S., Mumcuoglu K.Y. Protozoan and Microbial Pathogens of House Cats in the Province of Tekirdag in Western Turkey. Pathogens. 2021;10:1114. doi: 10.3390/pathogens10091114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Selim A., Alanazi A.D., Sazmand A., Otranto D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasit Vectors. 2021;14:175. doi: 10.1186/s13071-021-04670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Elhelw R., Elhariri M., Hamza D., Abuowarda M., Ismael E., Farag H. Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet. Res. 2021;17:49. doi: 10.1186/s12917-020-02733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ben Said M., Belkahia H., Alberti A., Abdi K., Zhioua M., Daaloul-Jedidi M., Messadi L. First molecular evidence of [i]Borrelia burgdorferi[/i] sensu lato in goats, sheep, cattle and camels in Tunisia. Ann. Agric. Environ. Med. 2016;23:442–447. doi: 10.5604/12321966.1219184. [DOI] [PubMed] [Google Scholar]
- 281.Dsouli N., Younsi-Kabachii H., Postic D., Nouira S., Gern L., Bouattour A. Reservoir role of lizard Psammodromus algirus in transmission cycle of Borrelia burgdorferi sensu lato (Spirochaetaceae) in Tunisia. J. Med. Entomol. 2006;43:737–742. doi: 10.1603/0022-2585(2006)43[737:rrolpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 282.Azzag N., Petit E., Gandoin C., Bouillin C., Ghalmi F., Haddad N., Boulouis H.J. Prevalence of select vector-borne pathogens in stray and client-owned dogs from Algiers. Comp. Immunol. Microbiol. Infect. Dis. 2015;38:1–7. doi: 10.1016/j.cimid.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 283.Laamari A., Azzag N., Tennah S., Derdour S.Y., China B., Bouabdallah R., Ghalmi F. Seroprevalence of Antibodies Against Anaplasma Phagocytophilum and Borrelia Burgdorferi in Horses (Equus caballus) from Northern Algeria. J. Vet. Res. 2020;64:413–419. doi: 10.2478/jvetres-2020-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lledo L., Gegundez M.I., Saz J.V., Beltran M. Screening of the prevalence of antibodies to Borrelia burgdorferi in Madrid province, Spain. Eur. J. Epidemiol. 2004;19:471–472. doi: 10.1023/b:ejep.0000027349.48337.cb. [DOI] [PubMed] [Google Scholar]
- 285.Escudero R., Barral M., Perez A., Vitutia M.M., Garcia-Perez A.L., Jimenez S., Sellek R.E., Anda P. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 2000;38:4026–4033. doi: 10.1128/JCM.38.11.4026-4033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Grillon A., Scherlinger M., Boyer P.H., De Martino S., Perdriger A., Blasquez A., Wipff J., Korganow A.S., Bonnard C., Cantagrel A., et al. Characteristics and clinical outcomes after treatment of a national cohort of PCR-positive Lyme arthritis. Semin. Arthritis Rheum. 2019;48:1105–1112. doi: 10.1016/j.semarthrit.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 287.Lenormand C., Jaulhac B., De Martino S., Barthel C., Lipsker D. Species of Borrelia burgdorferi complex that cause borrelial lymphocytoma in France. Br. J. Dermatol. 2009;161:174–176. doi: 10.1111/j.1365-2133.2009.09100.x. [DOI] [PubMed] [Google Scholar]
- 288.Del Giudice P., Freychet F., Kopec L., Fenollar F., Eldin C., Velin M., Hubiche T., Raoult D., Mediannikov O. Erythema Migrans Caused by Borrelia spielmanii, France. Emerg. Infect. Dis. 2023;29:2366–2369. doi: 10.3201/eid2911.230149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ciceroni L., Ciarrochi S., Ciervo A., Mondarini V., Guzzo F., Caruso G., Murgia R., Cinco M. Isolation and characterization of Borrelia burgdorferi sensu lato strains in an area of Italy where Lyme borreliosis is endemic. J. Clin. Microbiol. 2001;39:2254–2260. doi: 10.1128/JCM.39.6.2254-2260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Santino I., Berlutti F., Pantanella F., Sessa R., del Piano M. Detection of Borrelia burgdorferi sensu lato DNA by PCR in serum of patients with clinical symptoms of Lyme borreliosis. FEMS Microbiol. Lett. 2008;283:30–35. doi: 10.1111/j.1574-6968.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- 291.Sonnleitner S.T., Margos G., Wex F., Simeoni J., Zelger R., Schmutzhard E., Lass-Florl C., Walder G. Human seroprevalence against Borrelia burgdorferi sensu lato in two comparable regions of the eastern Alps is not correlated to vector infection rates. Ticks Tick-Borne Dis. 2015;6:221–227. doi: 10.1016/j.ttbdis.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 292.Strle F., Lusa L., Ruzic-Sabljic E., Maraspin V., Lotric Furlan S., Cimperman J., Ogrinc K., Rojko T., Videcnik Zorman J., Stupica D. Clinical characteristics associated with Borrelia burgdorferi sensu lato skin culture results in patients with erythema migrans. PLoS ONE. 2013;8:e82132. doi: 10.1371/journal.pone.0082132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gatzmann F., Metzler D., Krebs S., Blum H., Sing A., Takano A., Kawabata H., Fingerle V., Margos G., Becker N.S. NGS population genetics analyses reveal divergent evolution of a Lyme Borreliosis agent in Europe and Asia. Ticks Tick-Borne Dis. 2015;6:344–351. doi: 10.1016/j.ttbdis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 294.Maraspin V., Ruzic-Sabljic E., Strle F. Lyme borreliosis and Borrelia spielmanii. Emerg. Infect. Dis. 2006;12:1177. doi: 10.3201/eid1207.060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ruzic-Sabljic E., Zore A., Strle F. Characterization of Borrelia burgdorferi sensu lato isolates by pulsed-field gel electrophoresis after MluI restriction of genomic DNA. Res. Microbiol. 2008;159:441–448. doi: 10.1016/j.resmic.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 296.Maraspin V., Nahtigal Klevisar M., Ruzic-Sabljic E., Lusa L., Strle F. Borrelial Lymphocytoma in Adult Patients. Clin. Infect. Dis. 2016;63:914–921. doi: 10.1093/cid/ciw417. [DOI] [PubMed] [Google Scholar]
- 297.Burek V., Misic-Mayerus L., Maretic T. Antibodies to Borrelia burgdorferi in various population groups in Croatia. Scand. J. Infect. Dis. 1992;24:683–684. doi: 10.3109/00365549209054658. [DOI] [PubMed] [Google Scholar]
- 298.Situm M., Grahovac B., Markovic S., Lipozencic J., Poje G., Dobric I., Marinovic B., Bolanca-Bumber S., Misic-Majerus L. Detection and genotyping of Borrelia burgdorferi sensu lato by polymerase chain reaction. Croat. Med. J. 2000;41:47–53. [PubMed] [Google Scholar]
- 299.Mulic R., Antonijevic S., Klismanic Z., Ropac D., Lucev O. Epidemiological characteristics and clinical manifestations of Lyme borreliosis in Croatia. Mil. Med. 2006;171:1105–1109. doi: 10.7205/milmed.171.11.1105. [DOI] [PubMed] [Google Scholar]
- 300.Arapovic J., Skocibusic S., Grgic S., Nikolic J. The first evidence of lyme neuroborreliosis in southern bosnia and herzegovina. Case Rep. Infect. Dis. 2014;2014:231969. doi: 10.1155/2014/231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Karageorgou I., Koutantou M., Papadogiannaki I., Voulgari-Kokota A., Makka S., Angelakis E. Serological evidence of possible Borrelia afzelii lyme disease in Greece. New Microbes New Infect. 2022;46:100978. doi: 10.1016/j.nmni.2022.100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Onal U., Aytac Erdem H., Uyan Onal A., Resat Sipahi O. Systematic review of Lyme disease in Turkey. Trop. Dr. 2019;49:165–170. doi: 10.1177/0049475519843387. [DOI] [PubMed] [Google Scholar]
- 303.Gazi H., Ozkutuk N., Ecemis O., Atasoylu G., Koroglu G., Kurutepe S., Horasan G.D. Seroprevalence of West Nile virus, Crimean-Congo hemorrhagic fever virus, Francisella tularensis and Borrelia burgdorferi in rural population of Manisa, western Turkey. J. Vector Borne Dis. 2016;53:112–117. doi: 10.4103/0972-9062.184827. [DOI] [PubMed] [Google Scholar]
- 304.Kaya A.D., Parlak A.H., Ozturk C.E., Behcet M. Seroprevalence of Borrelia burgdorferi infection among forestry workers and farmers in Duzce, north-western Turkey. New Microbiol. 2008;31:203–209. [PubMed] [Google Scholar]
- 305.Abraham Z., Feuerman E.J., Rozenbaum M., Gluck Z. Lyme disease in Israel. J. Am. Acad. Dermatol. 1991;25:729. doi: 10.1016/s0190-9622(08)80681-0. [DOI] [PubMed] [Google Scholar]
- 306.Dworkin M.S., Schwan T.G., Anderson D.E., Jr., Borchardt S.M. Tick-borne relapsing fever. Infect. Dis. Clin. N. Am. 2008;22:449–468, viii. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Faccini-Martinez A.A., Silva-Ramos C.R., Santodomingo A.M., Ramirez-Hernandez A., Costa F.B., Labruna M.B., Munoz-Leal S. Historical overview and update on relapsing fever group Borrelia in Latin America. Parasit Vectors. 2022;15:196. doi: 10.1186/s13071-022-05289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cutler S.J. Relapsing Fever Borreliae: A Global Review. Clin. Lab. Med. 2015;35:847–865. doi: 10.1016/j.cll.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 309.Larsson C., Andersson M., Guo B.P., Nordstrand A., Hagerstrand I., Carlsson S., Bergstrom S. Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. J. Infect. Dis. 2006;194:1367–1374. doi: 10.1086/508425. [DOI] [PubMed] [Google Scholar]
- 310.Melkert P.W. Relapsing fever in pregnancy: Analysis of high-risk factors. Br. J. Obstet. Gynaecol. 1988;95:1070–1072. doi: 10.1111/j.1471-0528.1988.tb06516.x. [DOI] [PubMed] [Google Scholar]
- 311.Bryceson A.D., Parry E.H., Perine P.L., Warrell D.A., Vukotich D., Leithead C.S. Louse-borne relapsing fever. Q. J. Med. 1970;39:129–170. [PubMed] [Google Scholar]
- 312.Warrell D.A. Louse-borne relapsing fever (Borrelia recurrentis infection) Epidemiol. Infect. 2019;147:e106. doi: 10.1017/S0950268819000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kahlig P., Neumayr A., Paris D.H. Louse-borne relapsing fever-A systematic review and analysis of the literature: Part 2-Mortality, Jarisch-Herxheimer reaction, impact on pregnancy. PLoS Negl. Trop. Dis. 2021;15:e0008656. doi: 10.1371/journal.pntd.0008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Anda P., Sanchez-Yebra W., del Mar Vitutia M., Perez Pastrana E., Rodriguez I., Miller N.S., Backenson P.B., Benach J.L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet. 1996;348:162–165. doi: 10.1016/s0140-6736(96)02332-x. [DOI] [PubMed] [Google Scholar]
- 315.Wyplosz B., Mihaila-Amrouche L., Baixench M.T., Bigel M.L., Berardi-Grassias L., Fontaine C., Hornstein M., Izri A., Baranton G., Postic D. Imported tickborne relapsing fever, France. Emerg. Infect. Dis. 2005;11:1801–1803. doi: 10.3201/eid1111.050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kahlig P., Paris D.H., Neumayr A. Louse-borne relapsing fever-A systematic review and analysis of the literature: Part 1-Epidemiology and diagnostic aspects. PLoS Negl. Trop. Dis. 2021;15:e0008564. doi: 10.1371/journal.pntd.0008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ramos J.M., Malmierca E., Reyes F., Tesfamariam A. Results of a 10-year survey of louse-borne relapsing fever in southern Ethiopia: A decline in endemicity. Ann. Trop. Med. Parasitol. 2008;102:467–469. doi: 10.1179/136485908X300887. [DOI] [PubMed] [Google Scholar]
- 318.Ramos J.M., Malmierca E., Reyes F., Wolde W., Galata A., Tesfamariam A., Gorgolas M. Characteristics of louse-borne relapsing fever in Ethiopian children and adults. Ann. Trop. Med. Parasitol. 2004;98:191–196. doi: 10.1179/000349804225003136. [DOI] [PubMed] [Google Scholar]
- 319.Nordmann T., Feldt T., Bosselmann M., Tufa T.B., Lemma G., Holtfreter M., Haussinger D. Outbreak of Louse-Borne Relapsing Fever among Urban Dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am. J. Trop. Med. Hyg. 2018;98:1599–1602. doi: 10.4269/ajtmh.17-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Darcis G., Hayette M.P., Bontems S., Sauvage A.S., Meuris C., Van Esbroeck M., Leonard P. Louse-borne relapsing fever in a refugee from Somalia arriving in Belgium. J. Travel Med. 2016;23:taw009. doi: 10.1093/jtm/taw009. [DOI] [PubMed] [Google Scholar]
- 321.Osthoff M., Schibli A., Fadini D., Lardelli P., Goldenberger D. Louse-borne relapsing fever—Report of four cases in Switzerland, June-December 2015. BMC Infect Dis. 2016;16:210. doi: 10.1186/s12879-016-1541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Naddaf S.R., Ghazinezhad B., Kazemirad E., Cutler S.J. Relapsing fever causative agent in Southern Iran is a closely related species to East African borreliae. Ticks Tick-Borne Dis. 2017;8:882–886. doi: 10.1016/j.ttbdis.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 323.Raoult D., Birtles R.J., Montoya M., Perez E., Tissot-Dupont H., Roux V., Guerra H. Survey of three bacterial louse-associated diseases among rural Andean communities in Peru: Prevalence of epidemic typhus, trench fever, and relapsing fever. Clin. Infect. Dis. 1999;29:434–436. doi: 10.1086/520229. [DOI] [PubMed] [Google Scholar]
- 324.Brouqui P., Stein A., Dupont H.T., Gallian P., Badiaga S., Rolain J.M., Mege J.L., La Scola B., Berbis P., Raoult D. Ectoparasitism and vector-borne diseases in 930 homeless people from Marseilles. Medicine. 2005;84:61–68. doi: 10.1097/01.md.0000152373.07500.6e. [DOI] [PubMed] [Google Scholar]
- 325.Ly T.D.A., Louni M., Hoang V.T., Dao T.L., Badiaga S., Brouqui P., Tissot-Dupont H., Raoult D., Fournier P.E., Gautret P. Epidemiological serosurvey of vector-borne and zoonotic pathogens among homeless people living in shelters in Marseille: Cross-sectional one-day surveys (2005–2015) Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1663–1672. doi: 10.1007/s10096-020-03889-6. [DOI] [PubMed] [Google Scholar]
- 326.Ciervo A., Mancini F., di Bernardo F., Giammanco A., Vitale G., Dones P., Fasciana T., Quartaro P., Mazzola G., Rezza G. Louseborne Relapsing Fever in Young Migrants, Sicily, Italy, July–September 2015. Emerg. Infect. Dis. 2016;22:152–153. doi: 10.3201/eid2201.151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cutler S.J., Moss J., Fukunaga M., Wright D.J., Fekade D., Warrell D. Borrelia recurrentis characterization and comparison with relapsing-fever, Lyme-associated, and other Borrelia spp. Int. J. Syst. Bacteriol. 1997;47:958–968. doi: 10.1099/00207713-47-4-958. [DOI] [PubMed] [Google Scholar]
- 328.Munoz-Leal S., Faccini-Martinez A.A., Costa F.B., Marcili A., Mesquita E., Marques E.P., Jr., Labruna M.B. Isolation and molecular characterization of a relapsing fever Borrelia recovered from Ornithodoros rudis in Brazil. Ticks Tick-Borne Dis. 2018;9:864–871. doi: 10.1016/j.ttbdis.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 329.Assous M.V., Wilamowski A. Relapsing fever borreliosis in Eurasia--forgotten, but certainly not gone! Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009;15:407–414. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 330.Trape J.F., Diatta G., Arnathau C., Bitam I., Sarih M., Belghyti D., Bouattour A., Elguero E., Vial L., Mane Y., et al. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida) PLoS ONE. 2013;8:e78473. doi: 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schwan T.G., Hinnebusch B.J. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 332.Boyle W.K., Wilder H.K., Lawrence A.M., Lopez J.E. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl. Trop. Dis. 2014;8:e2767. doi: 10.1371/journal.pntd.0002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Johnson T.L., Landguth E.L., Stone E.F. Modeling Relapsing Disease Dynamics in a Host-Vector Community. PLoS Negl. Trop. Dis. 2016;10:e0004428. doi: 10.1371/journal.pntd.0004428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Barbour A.G. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect. Genet. Evol. 2014;27:551–558. doi: 10.1016/j.meegid.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Remesar S., Diaz P., Venzal J.M., Prieto A., Estrada-Pena A., Lopez C.M., Panadero R., Fernandez G., Diez-Banos P., Morrondo P. Longitudinal Study of Infection with Borrelia spp. in Questing Ticks from North-Western Spain. Vector Borne Zoonotic Dis. 2019;19:785–792. doi: 10.1089/vbz.2019.2442. [DOI] [PubMed] [Google Scholar]
- 336.Diaz P., Remesar S., Venzal J.M., Vazquez-Lopez M.E., Fernandez G., Lopez C., Diez-Banos P., Morrondo P., Panadero R. Occurrence of Borrelia and Borreliella species in Ixodes ricinus collected from roe deer in northwestern Spain. Med. Vet. Entomol. 2019;33:427–430. doi: 10.1111/mve.12364. [DOI] [PubMed] [Google Scholar]
- 337.Sanz-Aguilar A., Payo-Payo A., Rotger A., Yousfi L., Moutailler S., Beck C., Dumarest M., Igual J.M., Miranda M.A., Vinas Torres M., et al. Infestation of small seabirds by Ornithodoros maritimus ticks: Effects on chick body condition, reproduction and associated infectious agents. Ticks Tick-Borne Dis. 2020;11:101281. doi: 10.1016/j.ttbdis.2019.101281. [DOI] [PubMed] [Google Scholar]
- 338.Lejal E., Marsot M., Chalvet-Monfray K., Cosson J.F., Moutailler S., Vayssier-Taussat M., Pollet T. A three-years assessment of Ixodes ricinus-borne pathogens in a French peri-urban forest. Parasit Vectors. 2019;12:551. doi: 10.1186/s13071-019-3799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sevestre J., Diarra A.Z., Oumarou H.A., Durant J., Delaunay P., Parola P. Detection of emerging tick-borne disease agents in the Alpes-Maritimes region, southeastern France. Ticks Tick-Borne Dis. 2021;12:101800. doi: 10.1016/j.ttbdis.2021.101800. [DOI] [PubMed] [Google Scholar]
- 340.Grech-Angelini S., Stachurski F., Vayssier-Taussat M., Devillers E., Casabianca F., Lancelot R., Uilenberg G., Moutailler S. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 2020;67:745–757. doi: 10.1111/tbed.13393. [DOI] [PubMed] [Google Scholar]
- 341.Socolovschi C., Kernif T., Raoult D., Parola P. Borrelia, Rickettsia, and Ehrlichia species in bat ticks, France, 2010. Emerg. Infect. Dis. 2012;18:1966–1975. doi: 10.3201/eid1812.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Jumpertz M., Sevestre J., Luciani L., Houhamdi L., Fournier P.E., Parola P. Bacterial Agents Detected in 418 Ticks Removed from Humans during 2014–2021, France. Emerg. Infect. Dis. 2023;29:701–710. doi: 10.3201/eid2904.221572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Garcia-Vozmediano A., Krawczyk A.I., Sprong H., Rossi L., Ramassa E., Tomassone L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick-Borne Dis. 2020;11:101489. doi: 10.1016/j.ttbdis.2020.101489. [DOI] [PubMed] [Google Scholar]
- 344.Ravagnan S., Tomassone L., Montarsi F., Krawczyk A.I., Mastrorilli E., Sprong H., Milani A., Rossi L., Capelli G. First detection of Borrelia miyamotoi in Ixodes ricinus ticks from northern Italy. Parasit Vectors. 2018;11:130. doi: 10.1186/s13071-018-2713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sakakibara K., Sen E., Sato K., Kawabata H., Ohashi N., Masuzawa T. Detection and Characterization of the Emerging Relapsing Fever Pathogen, Borrelia miyamotoi, from the Ixodes ricinus Tick in the Rural Trakya (Thrace) Region of Northwestern Turkey. Vector Borne Zoonotic Dis. 2016;16:797–799. doi: 10.1089/vbz.2016.2012. [DOI] [PubMed] [Google Scholar]
- 346.Norte A.C., Harris D.J., Silveira D., Nunes C.S., Nuncio M.S., Martinez E.G., Gimenez A., de Sousa R., Lopes de Carvalho I., Perera A. Diversity of microorganisms in Hyalomma aegyptium collected from spur-thighed tortoise (Testudo graeca) in North Africa and Anatolia. Transbound. Emerg. Dis. 2022;69:1951–1962. doi: 10.1111/tbed.14188. [DOI] [PubMed] [Google Scholar]
- 347.Safdie G., Farrah I.Y., Yahia R., Marva E., Wilamowski A., Sawalha S.S., Wald N., Schmiedel J., Moter A., Gobel U.B., et al. Molecular characterization of Borrelia persica, the agent of tick borne relapsing fever in Israel and the Palestinian Authority. PLoS ONE. 2010;5:e14105. doi: 10.1371/journal.pone.0014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Assous M.V., Wilamowski A., Bercovier H., Marva E. Molecular characterization of tickborne relapsing fever Borrelia, Israel. Emerg. Infect. Dis. 2006;12:1740–1743. doi: 10.3201/eid1211.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kleinerman G., Eshed T., Nachum-Biala Y., King R., Baneth G. Transmission of the Human Relapsing Fever Spirochete Borrelia persica by the Argasid Tick Ornithodoros tholozani Involves Blood Meals from Wildlife Animal Reservoirs and Mainly Transstadial Transfer. Appl. Environ. Microbiol. 2021;87:e03117-20. doi: 10.1128/AEM.03117-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Helmy N. Seasonal abundance of Ornithodoros (O.) savignyi and prevalence of infection with Borrelia spirochetes in Egypt. J. Egypt. Soc. Parasitol. 2000;30:607–619. [PubMed] [Google Scholar]
- 351.Abdullah H., Aboelsoued D., Farag T.K., Abdel-Shafy S., Abdel Megeed K.N., Parola P., Raoult D., Mediannikov O. Molecular characterization of some equine vector-borne diseases and associated arthropods in Egypt. Acta Trop. 2022;227:106274. doi: 10.1016/j.actatropica.2021.106274. [DOI] [PubMed] [Google Scholar]
- 352.Khalil G.M., Helmy N., Hoogstraal H., el-Said A. Seasonal dynamics of Ornithodoros (Pavlovskyella) erraticus (Acari: Ixodoidea: Argasidae) and the spirochete Borrelia crocidurae in Egypt. J. Med. Entomol. 1984;21:536–539. doi: 10.1093/jmedent/21.5.536. [DOI] [PubMed] [Google Scholar]
- 353.Balti G., Galon C., Derghal M., Souguir H., Guerbouj S., Rhim A., Chemkhi J., Guizani I., Bouattour A., Moutailler S., et al. Atelerix algirus, the North African Hedgehog: Suitable Wild Host for Infected Ticks and Fleas and Reservoir of Vector-Borne Pathogens in Tunisia. Pathogens. 2021;10:953. doi: 10.3390/pathogens10080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Bouattour A., Garnier M., M’Ghirbi Y., Sarih M., Gern L., Ferquel E., Postic D., Cornet M. Borrelia crocidurae infection of Ornithodoros erraticus (Lucas, 1849) ticks in Tunisia. Vector Borne Zoonotic Dis. 2010;10:825–830. doi: 10.1089/vbz.2009.0151. [DOI] [PubMed] [Google Scholar]
- 355.Lafri I., El Hamzaoui B., Bitam I., Leulmi H., Lalout R., Mediannikov O., Chergui M., Karakellah M., Raoult D., Parola P. Detection of relapsing fever Borrelia spp., Bartonella spp. and Anaplasmataceae bacteria in argasid ticks in Algeria. PLoS Negl. Trop. Dis. 2017;11:e0006064. doi: 10.1371/journal.pntd.0006064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ouchene N., Nebbak A., Ouchene-Khelifi N.A., Dahmani A., Zeroual F., Khelef D., Bitam I., Benakhla A., Parola P. Molecular detection of avian spirochete Borrelia anserina in Argas persicus ticks in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020;68:101408. doi: 10.1016/j.cimid.2019.101408. [DOI] [PubMed] [Google Scholar]
- 357.Diatta G., Souidi Y., Granjon L., Arnathau C., Durand P., Chauvancy G., Mane Y., Sarih M., Belghyti D., Renaud F., et al. Epidemiology of tick-borne borreliosis in Morocco. PLoS Negl. Trop. Dis. 2012;6:e1810. doi: 10.1371/journal.pntd.0001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Souidi Y., Boudebouch N., Ezikouri S., Belghyti D., Trape J.F., Sarih M. Borrelia crocidurae in Ornithodoros ticks from northwestern Morocco: A range extension in relation to climatic change? J. Vector Ecol. 2014;39:316–320. doi: 10.1111/jvec.12106. [DOI] [PubMed] [Google Scholar]
- 359.Johnson T.L., Fischer R.J., Raffel S.J., Schwan T.G. Host associations and genomic diversity of Borrelia hermsii in an endemic focus of tick-borne relapsing fever in western North America. Parasit Vectors. 2016;9:575. doi: 10.1186/s13071-016-1863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Han S., Hickling G.J., Tsao J.I. High Prevalence of Borrelia miyamotoi among Adult Blacklegged Ticks from White-Tailed Deer. Emerg. Infect. Dis. 2016;22:316–318. doi: 10.3201/eid2202.151218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Larsson C., Lundqvist J., van Rooijen N., Bergstrom S. A novel animal model of Borrelia recurrentis louse-borne relapsing fever borreliosis using immunodeficient mice. PLoS Negl. Trop. Dis. 2009;3:e522. doi: 10.1371/journal.pntd.0000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.McCall P.J., Hume J.C., Motshegwa K., Pignatelli P., Talbert A., Kisinza W. Does tick-borne relapsing fever have an animal reservoir in East Africa? Vector Borne Zoonotic Dis. 2007;7:659–666. doi: 10.1089/vbz.2007.0151. [DOI] [PubMed] [Google Scholar]
- 363.Meri T., Cutler S.J., Blom A.M., Meri S., Jokiranta T.S. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect. Immun. 2006;74:4157–4163. doi: 10.1128/IAI.00007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Margos G., Pantchev N., Globokar M., Lopez J., Rodon J., Hernandez L., Herold H., Salas N., Civit A., Fingerle V. First Cases of Natural Infections with Borrelia hispanica in Two Dogs and a Cat from Europe. Microorganisms. 2020;8:1251. doi: 10.3390/microorganisms8081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Cosson J.F., Michelet L., Chotte J., Le Naour E., Cote M., Devillers E., Poulle M.L., Huet D., Galan M., Geller J., et al. Genetic characterization of the human relapsing fever spirochete Borrelia miyamotoi in vectors and animal reservoirs of Lyme disease spirochetes in France. Parasit Vectors. 2014;7:233. doi: 10.1186/1756-3305-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Celebi B., Yeni D.K., Yilmaz Y., Matur F., Babur C., Oktem M.A., Sozen M., Karatas A., Raoult D., Mediannikov O., et al. Borrelia miyamotoi in wild rodents from four different regions of Turkey. Ticks Tick-Borne Dis. 2023;14:102143. doi: 10.1016/j.ttbdis.2023.102143. [DOI] [PubMed] [Google Scholar]
- 367.Kleinerman G., King R., Nachum-Biala Y., Baneth G. Borrelia persica infection in rock hyraxes. Ticks Tick-Borne Dis. 2018;9:382–388. doi: 10.1016/j.ttbdis.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 368.Baneth G., Nachum-Biala Y., Halperin T., Hershko Y., Kleinerman G., Anug Y., Abdeen Z., Lavy E., Aroch I., Straubinger R.K. Borrelia persica infection in dogs and cats: Clinical manifestations, clinicopathological findings and genetic characterization. Parasit Vectors. 2016;9:244. doi: 10.1186/s13071-016-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Baneth G., Dvorkin A., Ben-Shitrit B., Kleinerman G., Salant H., Straubinger R.K., Nachum-Biala Y. Infection and seroprevalence of Borrelia persica in domestic cats and dogs in Israel. Parasit Vectors. 2022;15:102. doi: 10.1186/s13071-022-05223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Shwartz D., Nachum-Biala Y., Oren S., Aharoni K., Edery N., Moss L., King R., Lapid R., Straubinger R.K., Baneth G. Borrelia persica infection in wild carnivores in Israel: Molecular characterization and new potential reservoirs. Parasit Vectors. 2023;16:337. doi: 10.1186/s13071-023-05953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Abdullah H., Elbayoumy M.K., Allam A.M., Ashry H.M., Abdel-Shafy S. Molecular epidemiology of certain vector-borne bacterial microorganisms in domestic animals and their ectoparasites in Egypt. Trop. Anim. Health Prod. 2021;53:484. doi: 10.1007/s11250-021-02911-z. [DOI] [PubMed] [Google Scholar]
- 372.Abdullah H., Amanzougaghene N., Dahmana H., Louni M., Raoult D., Mediannikov O. Multiple vector-borne pathogens of domestic animals in Egypt. PLoS Negl. Trop. Dis. 2021;15:e0009767. doi: 10.1371/journal.pntd.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.El-Alfy E.S., Abbas I., Baghdadi H.B., El-Sayed S.A.E., Ji S., Rizk M.A. Molecular Epidemiology and Species Diversity of Tick-Borne Pathogens of Animals in Egypt: A Systematic Review and Meta-Analysis. Pathogens. 2022;11:912. doi: 10.3390/pathogens11080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Fichet-Calvet E., Jomaa I., Ben Ismail R., Ashford R.W. Patterns of infection of haemoparasites in the fat sand rat, Psammomys obesus, in Tunisia, and effect on the host. Ann. Trop. Med. Parasitol. 2000;94:55–68. doi: 10.1080/00034980057617. [DOI] [PubMed] [Google Scholar]
- 375.Sadeddine R., Diarra A.Z., Laroche M., Mediannikov O., Righi S., Benakhla A., Dahmana H., Raoult D., Parola P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick-Borne Dis. 2020;11:101330. doi: 10.1016/j.ttbdis.2019.101330. [DOI] [PubMed] [Google Scholar]
- 376.Aouadi A., Leulmi H., Boucheikhchoukh M., Benakhla A., Raoult D., Parola P. Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017;50:34–39. doi: 10.1016/j.cimid.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 377.Diatta G., Duplantier J.M., Granjon L., Ba K., Chauvancy G., Ndiaye M., Trape J.F. Borrelia infection in small mammals in West Africa and its relationship with tick occurrence inside burrows. Acta Trop. 2015;152:131–140. doi: 10.1016/j.actatropica.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 378.Castilla-Guerra L., Alvarez-Suero J., Del Carmen Fernandez-Moreno M., Fontana E.R. Tick-borne relapsing fever: Conjunctival haemorrhages. BMJ Case Rep. 2009;2009:bcr0620080114. doi: 10.1136/bcr.06.2008.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lopez-Cortes L., Lozano de Leon F., Gomez-Mateos J.M., Sanchez-Porto A., Obrador C. Tick-borne relapsing fever in intravenous drug abusers. J. Infect. Dis. 1989;159:804. doi: 10.1093/infdis/159.4.804. [DOI] [PubMed] [Google Scholar]
- 380.Dominguez M.C., Vergara S., Gomez M.C., Roldan M.E. Epidemiology of Tick-Borne Relapsing Fever in Endemic Area, Spain. Emerg. Infect. Dis. 2020;26:849–856. doi: 10.3201/eid2605.190745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Castilla-Guerra L., Marin-Martin J., Colmenero-Camacho M.A. Tick-Borne Relapsing Fever, Southern Spain, 2004–2015. Emerg. Infect. Dis. 2016;22:2217–2219. doi: 10.3201/eid2212.160870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Lacout A., Mas M., Pajaud J., Perronne V., Lequette Y., Franck M., Perronne C. Real time micro-organisms PCR in 104 patients with polymorphic signs and symptoms that may be related to a tick bite. Eur. J. Microbiol. Immunol. 2021;11:62–75. doi: 10.1556/1886.2021.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Malincarne L., Schiaroli E., Ciervo A., Scaglione V., Paciaroni M., Mancini F., Paglia M.G., Cardaci S., Pasticci M.B., Francisci D., et al. Meningitis with cranial polyneuritis and cavernous sinus thrombosis by Borrelia crocidurae: First autochthonous case in Europe. Int. J. Infect. Dis. 2019;82:30–32. doi: 10.1016/j.ijid.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 384.Billiet A., Vanderschueren S., Lagrou K., Pilate T., Fournier P.E., Luciani L., Henckaerts L. Tick borne relapsing fever after travelling to a Greek island. J. Travel. Med. 2022;29:taab073. doi: 10.1093/jtm/taab073. [DOI] [PubMed] [Google Scholar]
- 385.Gambles R.M., Coghill N.F. Relapsing fever in Cyprus. Ann. Trop. Med. Parasitol. 1948;42:288–303. doi: 10.1080/00034983.1948.11685378. [DOI] [PubMed] [Google Scholar]
- 386.Wood R.C., Dixon K.C. Tick-borne Relapsing Fever. Br. Med. J. 1945;2:526–528. doi: 10.1136/bmj.2.4424.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Simon J.W. Tick borne relapsing fever imported into the United Kingdom. J. R. Army Med. Corps. 1985;131:65–67. doi: 10.1136/jramc-131-02-02. [DOI] [PubMed] [Google Scholar]
- 388.Moran-Gilad J., Levine H., Schwartz E., Bartal C., Huerta-Hartal M., Schwaber M.J., Ostfeld I. Postexposure prophylaxis of tick-borne relapsing fever: Lessons learned from recent outbreaks in Israel. Vector Borne Zoonotic Dis. 2013;13:791–797. doi: 10.1089/vbz.2013.1347. [DOI] [PubMed] [Google Scholar]
- 389.Fuchs I., Tarabin S., Kafka M. Relapsing Fever: Diagnosis Thanks to a Vigilant Hematology Laboratory. Vector Borne Zoonotic Dis. 2015;15:446–448. doi: 10.1089/vbz.2014.1764. [DOI] [PubMed] [Google Scholar]
- 390.Yagupsky P., Moses S. Neonatal Borrelia species infection (relapsing fever) Am. J. Dis. Child. 1985;139:74–76. doi: 10.1001/archpedi.1985.02140030076034. [DOI] [PubMed] [Google Scholar]
- 391.Koton Y., Bisharat N. Tick-Borne Relapsing Fever with Severe Jarisch-Herxheimer Reaction. Isr. Med. Assoc. J. 2018;20:62–63. [PubMed] [Google Scholar]
- 392.Hasin T., Davidovitch N., Cohen R., Dagan T., Romem A., Orr N., Klement E., Lubezky N., Kayouf R., Sela T., et al. Postexposure treatment with doxycycline for the prevention of tick-borne relapsing fever. N. Engl. J. Med. 2006;355:148–155. doi: 10.1056/NEJMoa053884. [DOI] [PubMed] [Google Scholar]
- 393.Hashavya S., Gross I., Gross M., Hurvitz N., Weiser G., Temper V., Megged O. Tickborne Relapsing Fever, Jerusalem, Israel, 2004-2018. Emerg. Infect. Dis. 2020;26:2420–2423. doi: 10.3201/eid2610.181988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Halperin T., Orr N., Cohen R., Hasin T., Davidovitch N., Klement E., Kayouf R., Baneth G., Cohen D., Yavzori M. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (GlpQ) gene. Acta Trop. 2006;98:189–195. doi: 10.1016/j.actatropica.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 395.Yossepowitch O., Gottesman T., Schwartz-Harari O., Soroksky A., Dan M. Aseptic meningitis and adult respiratory distress syndrome caused by Borrelia persica. Infection. 2012;40:695–697. doi: 10.1007/s15010-012-0296-8. [DOI] [PubMed] [Google Scholar]
- 396.Dewar H.A., Walmsley R. Relapsing fever with nephritis and subarachnoid haemorrhage. Lancet. 1945;2:630. doi: 10.1016/s0140-6736(45)90763-x. [DOI] [PubMed] [Google Scholar]
- 397.Coghill N.F., Lawrence J., Ballantine I.D. Relapsing fever in Cyrenaica. Br. Med. J. 1947;1:637–640. doi: 10.1136/bmj.1.4505.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Leen I., Bruynseels P., Mukadi B.K., van Oort M., van den Akker M. A 13-year old girl with pancytopenia at the presentation of a Borrelia hispanica infection: A case report and review of the literature. J. Med. Case Rep. 2017;11:51. doi: 10.1186/s13256-017-1225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sarih M., Garnier M., Boudebouch N., Bouattour A., Rihani A., Hassar M., Gern L., Postic D., Cornet M. Borrelia hispanica relapsing fever, Morocco. Emerg. Infect. Dis. 2009;15:1626–1629. doi: 10.3201/eid1510.090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Goutier S., Ferquel E., Pinel C., Bosseray A., Hoen B., Couetdic G., Bourahoui A., Lapostolle C., Pelloux H., Garnier M., et al. Borrelia crocidurae meningoencephalitis, West Africa. Emerg. Infect. Dis. 2013;19:301–304. doi: 10.3201/eid1902.121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Gras E., Bailly E., Le Brun C., Lemaignen A., Lanotte P. Borrelia crocidurae tick-borne relapsing fever upon return from Senegal. Med. Mal. Infect. 2019;49:624–625. doi: 10.1016/j.medmal.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 402.Guiheneuf E., Desjardins N., Guiheneuf R. It is not always malaria: Diagnosis of Borrelia recurrent fever on blood smear. Ann. Biol. Clin. 2018;76:118–119. doi: 10.1684/abc.2017.1320. [DOI] [PubMed] [Google Scholar]
- 403.Million M., Cazorla C., Doudier B., La Scola B., Parola P., Drancourt M., Brouqui P. Molecular identification of Borrelia crocidurae in a patient returning from Senegal. BMJ Case Rep. 2009;2009:bcr0620080298. doi: 10.1136/bcr.06.2008.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Colin de Verdiere N., Hamane S., Assous M.V., Sertour N., Ferquel E., Cornet M. Tickborne relapsing fever caused by Borrelia persica, Uzbekistan and Tajikistan. Emerg. Infect. Dis. 2011;17:1325–1327. doi: 10.3201/eid1707.101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Cutuli S.L., De Pascale G., Spanu T., Dell’Anna A.M., Bocci M.G., Pallavicini F., Mancini F., Ciervo A., Antonelli M. Lice, rodents, and many hopes: A rare disease in a young ref. Crit. Care. 2017;21:81. doi: 10.1186/s13054-017-1666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Antinori S., Mediannikov O., Corbellino M., Grande R., Parravicini C., Bestetti G., Longhi E., Ricaboni D., Ehounoud C.B., Fenollar F., et al. Louse-Borne Relapsing Fever (Borrelia recurrentis) in a Somali Refugee Arriving in Italy: A Re-emerging Infection in Europe? PLoS Negl. Trop. Dis. 2016;10:e0004522. doi: 10.1371/journal.pntd.0004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Antinori S., Tonello C., Edouard S., Parravicini C., Gastaldi D., Grande R., Milazzo L., Ricaboni D., Fenollar F., Raoult D., et al. Diagnosis of Louse-Borne Relapsing Fever despite Negative Microscopy in Two Asylum Seekers from Eastern Africa. Am. J. Trop. Med. Hyg. 2017;97:1669–1672. doi: 10.4269/ajtmh.17-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Colomba C., Scarlata F., Di Carlo P., Giammanco A., Fasciana T., Trizzino M., Cascio A. Fourth case of louse-borne relapsing fever in Young Migrant, Sicily, Italy, December 2015. Mini Review Article. Public Health. 2016;139:22–26. doi: 10.1016/j.puhe.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 409.Zammarchi L., Antonelli A., Bartolini L., Pecile P., Trotta M., Rogasi P.G., Santini M.G., Dilaghi B., Grifoni S., Rossolini G.M., et al. Louse-Borne Relapsing Fever with Meningeal Involvement in an Immigrant from Somalia to Italy, October 2015. Vector Borne Zoonotic Dis. 2016;16:352–355. doi: 10.1089/vbz.2015.1928. [DOI] [PubMed] [Google Scholar]
- 410.Lucchini A., Lipani F., Costa C., Scarvaglieri M., Balbiano R., Carosella S., Calcagno A., Audagnotto S., Barbui A.M., Brossa S., et al. Louseborne Relapsing Fever among East African Refugees, Italy, 2015. Emerg. Infect. Dis. 2016;22:298–301. doi: 10.3201/eid2202.151768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Grecchi C., Zanotti P., Pontarelli A., Chiari E., Tomasoni L.R., Gulletta M., Barbui A., Caligaris S., Matteelli A., Castelli F. Louse-borne relapsing fever in a refugee from Mali. Infection. 2017;45:373–376. doi: 10.1007/s15010-017-0987-2. [DOI] [PubMed] [Google Scholar]
- 412.Tordini G., Giaccherini R., Corbisiero R., Zanelli G. Relapsing fever in a traveller from Senegal: Determination of Borrelia species using molecular methods. Trans. R. Soc. Trop. Med. Hyg. 2006;100:992–994. doi: 10.1016/j.trstmh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 413.Nitzan O., Blum A., Marva E., Katz A., Tzadok B.S., Nachum-Biala Y., Baneth G., Peretz A. Case Report: Infectious Diseases in Pilgrims Visiting the Holy Land. Am. J. Trop. Med. Hyg. 2017;97:611–614. doi: 10.4269/ajtmh.17-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Burn L., Pilz A., Vyse A., Gutierrez Rabá A.V., Angulo F.J., Tran T.M.P., Fletcher M.A., Gessner B.D., Moïsi J.C., Stark J.H. Seroprevalence of Lyme Borreliosis in Europe: Results from a Systematic Literature Review (2005–2020) Vector Borne Zoonotic Dis. 2023;23:195–220. doi: 10.1089/vbz.2022.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Montero E., Folgueras M., Rodriguez-Perez M., Perez-Ls L., Diaz-Arias J., Meana M., Revuelta B., Haapasalo K., Collazos J., Asensi V., et al. Retrospective study of the epidemiological risk and serological diagnosis of human babesiosis in Asturias, Northwestern Spain. Parasites Vectors. 2023;16:195. doi: 10.1186/s13071-023-05817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Fernandez-Jorge B., Almagro-Sanchez M., Escudero-Nieto R., Fonseca-Capdevila E. Erythema migrans due to Borrelia afzelii. Med. Clin. 2006;126:237–238. doi: 10.1157/13084875. [DOI] [PubMed] [Google Scholar]
- 417.Rigaud E., Jaulhac B., Garcia-Bonnet N., Hunfeld K.-P., Femenia F., Huet D., Goulvestre C., Vaillant V., Deffontaines G., Abadia-Benoist G. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clin. Microbiol. Infect. 2016;22:735.e1–735.e9. doi: 10.1016/j.cmi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 418.Jaulhac B., Chary-Valckenaere I., Sibilia J., Javier R.M., Piemont Y., Kuntz J.L., Monteil H., Pourel J. Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with lyme arthritis. Arthritis Rheum. 1996;39:736–745. doi: 10.1002/art.1780390505. [DOI] [PubMed] [Google Scholar]
- 419.Riescher S., Dos Santos A., Lecomte R., Lenoble C., Guillon B. A case report of unilateral cerebral vasculitis in adults: Keep in mind Lyme neuroborreliosis. BMC Infect. Dis. 2023;23:283. doi: 10.1186/s12879-023-08259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Bernard A., Kodjikian L., Abukhashabh A., Roure-Sobas C., Boibieux A., Denis P., Broussolle C., Seve P. Diagnosis of Lyme-associated uveitis: Value of serological testing in a tertiary centre. Br. J. Ophthalmol. 2018;102:369–372. doi: 10.1136/bjophthalmol-2017-310251. [DOI] [PubMed] [Google Scholar]
- 421.Jeantin L., Rodriguez-Regent C., De Martino S., Gayraud M., Cosserat J. Intrathecal antibody kinetics in neuroborreliosis: A report on three cases and a literature review. Infect. Dis. Now. 2021;51:627–629. doi: 10.1016/j.idnow.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 422.Christiann F., Rayet P., Patey O., Ngueodjibaye D.B., Theron-le Gargasson J.-F., Lafaix C. Lyme borreliosis in central France: A sero-epidemiologic examination involving hunters. Eur. J. Epidemiol. 1997;13:855. doi: 10.1023/a:1007420008690. [DOI] [PubMed] [Google Scholar]
- 423.Christiann F., Rayet P., Ngueodjibaye D.B., Patey O., Godefroy A., Klein J., Lapegue R., Theron-Le Gargasson J.-F., Godfroid E., Lafaix C. Endemic level of Lyme borreliosis in a region of central France: A sero-epidemiologic examination involving blood donors. Eur. J. Epidemiol. 1997;13:361–362. doi: 10.1023/a:1007331421763. [DOI] [PubMed] [Google Scholar]
- 424.Guet-Revillet H., Levy C., Vallet C., Maghraoui-Slim V., Dommergues M.-A., Hentgen V., Paget C., Laugel V., Cohen R., Ferroni A. Lyme neuroborreliosis in children: Report of nine cases and a review of the literature. Arch. Pediatr. 2019;26:133–137. doi: 10.1016/j.arcped.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 425.Gibaud M., Pauvert O., Gueden S., Durigneux J., Van Bogaert P. Opsoclonus in a child with neuroborreliosis: Case report and review of the literature. Arch. Pediatr. 2019;26:118–119. doi: 10.1016/j.arcped.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 426.Mereaux J.-L., Hebant B., Magne N., Quesney G., Lefaucheur R. Bilateral facial palsy in an older person. Age Ageing. 2020;49:887–888. doi: 10.1093/ageing/afaa082. [DOI] [PubMed] [Google Scholar]
- 427.Nubling M., Rieger M.A., Batsford S., Wagner M., Wertenschlag E., Hofmann F. Seroprevalence of infection with Borrelia burgdorferi s. l. in two adjacent regions of eastern France and southwestern Germany. Int. J. Med. Microbiol. 2002;291((Suppl. 33)):218. doi: 10.1016/s1438-4221(02)80057-1. [DOI] [PubMed] [Google Scholar]
- 428.Sevestre J., Benichou A., Rio V., Delaunay P., Gonfrier G., Martaresche C., Carlo V., Nakam S., Mondain V., Carles M., et al. Emergence of Lyme Disease on the French Riviera, a Retrospective Survey. Front. Med. 2022;9:737854. doi: 10.3389/fmed.2022.737854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Letrilliart L., Ragon B., Hanslik T., Flahault A. Lyme disease in France: A primary care-based prospective study. Epidemiol. Infect. 2005;133:935–942. doi: 10.1017/s0950268805004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Frey M., Jaulhac B., Piemont Y., Marcellin L., Boohs P.-M., Vautravers P., Jesel M., Kuntz J.-L., Monteil H., Sibilia J. Detection of Borrelia burgdorferi DNA in muscle of patients with chronic myalgia related to Lyme disease. Am. J. Med. 1998;104:591–594. doi: 10.1016/s0002-9343(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 431.Mercier G., Burckel A., Lucotte G. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in urine specimens of patients with erythema migrans lesions. Mol. Cell. Probes. 1997;11:89–94. doi: 10.1006/mcpr.1996.0090. [DOI] [PubMed] [Google Scholar]
- 432.Christiann F., Rayet P., Patey O., Lafaix C. Epidemiology of lyme disease in France: Lyme borreliosis in the region of Berry sud: A six year retrospective. Eur. J. Epidemiol. 1996;12:479–483. doi: 10.1007/bf00144000. [DOI] [PubMed] [Google Scholar]
- 433.Dhôte R., Basse-Guerineau A.L., Beaumesnil V., Christoforov B., Assous M.V. Full spectrum of clinical, serological, and epidemiological features of complicated forms of Lyme borreliosis in the Paris, France, area. Eur. J. Clin. Microbiol. Infect. Dis. 2000;19:809–815. doi: 10.1007/s100960000391. [DOI] [PubMed] [Google Scholar]
- 434.Arzouni J.P., Laveran M., Beytout J., Ramousse O., Raoult D. Comparison of western blot and microimmunofluorescence as tools for lyme disease seroepidemiology. Eur. J. Epidemiol. 1993;9:269–273. doi: 10.1007/bf00146262. [DOI] [PubMed] [Google Scholar]
- 435.Septfons A., Rigaud E., Benezet L., Velay A., Zilliox L., Baldinger L., Gonzalez G., Figoni J., de Valk H., Deffontaines G., et al. Seroprevalence for Borrelia burgdorferi sensu lato and tick-borne encephalitis virus antibodies and associated risk factors among forestry workers in northern France, 2019 to 2020. Eurosurveillance. 2023;28 doi: 10.2807/1560-7917.es.2023.28.32.2200961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Bonnet C., Figoni J., Souty C., Septfons A., de Martino S., de Valk H., Fournier L., Hanslik T., Jaulhac B., Blanchon T. Prevalence and factors associated with a prescription of a Lyme borreliosis serology for erythema migrans diagnosis in general practice: A study from the French sentinel network, 2009–2020. BMC Prim. Care. 2023;24:163. doi: 10.1186/s12875-023-02108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Zhioua E., Rodhain F., Binet P., Perez-Eid C. Prevalence of antibodies to Borrelia burgdorferi in forestry workers of Ile de France, France. Eur. J. Epidemiol. 1997;13:959–962. doi: 10.1023/a:1007465305193. [DOI] [PubMed] [Google Scholar]
- 438.Corre C., Coiffier G., Le Goff B., Ferreyra M., Guennic X., Patrat-Delon S., Degeilh B., Albert J.-D., Tattevin P. Lyme arthritis in Western Europe: A multicentre retrospective study. Eur. J. Clin. Microbiol. Infect. Dis. 2022;41:21–27. doi: 10.1007/s10096-021-04334-y. [DOI] [PubMed] [Google Scholar]
- 439.Jaulhac B., Heller R., Limbach F.X., Hansmann Y., Lipsker D., Monteil H., Sibilia J., Piemont Y. Direct Molecular Typing of Borrelia burgdorferi Sensu Lato Species in Synovial Samples from Patients with Lyme Arthritis. J. Clin. Microbiol. 2000;38:1895–1900. doi: 10.1128/jcm.38.5.1895-1900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Gocko X., Lenormand C., Lemogne C., Bouiller K., Gehanno J.-F., Rabaud C., Perrot S., Eldin C., de Broucker T., Roblot F., et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies. Med. Mal. Infect. 2019;49:296–317. doi: 10.1016/j.medmal.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 441.Assous M.V., Postic D., Paul G., Nevot P., Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur. J. Clin. Microbiol. Infect. Dis. 1993;12:261–268. doi: 10.1007/bf01967256. [DOI] [PubMed] [Google Scholar]
- 442.Bauvin O., Schmutz J.-L., De Martino S., Busato T., Cribier B., Barbaud A., Wahl D., Bursztejn A.-C. A foot tumour as late cutaneous Lyme borreliosis: A new entity? Br. J. Dermatol. 2017;177:1127–1130. doi: 10.1111/bjd.15633. [DOI] [PubMed] [Google Scholar]
- 443.del Giudice P., Reverte M., Giraudon E., Durant J., Ahmed Abdoulah S., Jaulhac B., Poirier J.-P. First case of documented Lyme borreliosis in the Alpes-Maritimes department of South-Eastern France: Erythema chronicum migrans associated with Borrelia afzelii. Ann. Dermatol. Venereol. 2022;149:146–147. doi: 10.1016/j.annder.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 444.Gallais F., De Martino S.J., Sauleau E.A., Hansmann Y., Lipsker D., Lenormand C., Talagrand-Reboul E., Boyer P.H., Boulanger N., Jaulhac B., et al. Multilocus sequence typing of clinica Borreliella afzelii strains: Population structure and differential ability to disseminate in humans. Parasites Vectors. 2018;11:374. doi: 10.1186/s13071-018-2938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Lenormand C., Jaulhac B., Debarbieux S., Dupin N., Granel-Brocard F., Adamski H., Barthel C., Cribier B., Lipsker D. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans [ACA]): A prospective study of 20 culture- and/or polymerase chain reaction (PCR)-documented cases. J. Am. Acad. Dermatol. 2016;74:685–692. doi: 10.1016/j.jaad.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 446.Coipan E.C., Jahfari S., Fonville M., Oei G.A., Spanjaard L., Takumi K., Hovius J.W., Sprong H. Imbalanced presence of Borrelia burgdorferi s.l. multilocus sequence types in clinical manifestations of Lyme borreliosis. Infect. Genet. Evol. 2016;42:66–76. doi: 10.1016/j.meegid.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 447.Jacquot M., Abrial D., Gasqui P., Bord S., Marsot M., Masseglia S., Pion A., Poux V., Zilliox L., Chapuis J.-L., et al. Multiple independent transmission cycles of a tick-borne pathogen within a local host community. Sci. Rep. 2016;6:31273. doi: 10.1038/srep31273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Eiferman V., Guenno G.L., Boiret-Dupre N., Barres B., Luciani L., Fournier P.E. Atypical Borrelia garinii infection in an immunocompromised patient mimicking high-grade lymphoma. Int. J. Infect. Dis. 2022;121:102–104. doi: 10.1016/j.ijid.2022.04.062. [DOI] [PubMed] [Google Scholar]
- 449.Duffau P., Korbi S., Guillotin V., Talagrand-Reboul E., Menard A., Peuchant O. An unexpected case of Borrelia garinii liver infection. Ann. Clin. Microbiol. Antimicrob. 2022;21:15. doi: 10.1186/s12941-022-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Limbach F.X., Jaulhac B., Puechal X., Monteil H., Kuntz J.L., Piemont Y., Sibilia J. Treatment resistant Lyme arthritis caused by Borrelia garinii. Ann. Rheum. Dis. 2001;60:284–286. doi: 10.1136/ard.60.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Calderaro A., Montecchini S., Gorrini C., Piccolo G., Chezzi C., Dettori G. Presence of anti-Borrelia burgdorferi antibodies and Borrelia burgdorferi sensu lato DNA in samples of subjects in an area of the Northern Italy in the period 2002–2008. Diagn. Microbiol. Infect. Dis. 2011;70:455–460. doi: 10.1016/j.diagmicrobio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 452.Santino I., Iori A., Sessa R., Sulli C., Favia G., Del Piano M. Borrelia burgdorferi s.l. and Ehrlichia chaffeensis in the National Park of Abruzzo. FEMS Microbiol. Lett. 1998;164:1–6. doi: 10.1111/j.1574-6968.1998.tb13059.x. [DOI] [PubMed] [Google Scholar]
- 453.Pauluzzi P., Bonin S., Gonzalez Inchaurraga M.A., Stanta G., Trevisan G. Detection of Spirochaetal DNA Simultaneously in Skin Biopsies, Peripheral Blood and Urine from Patients with Erythema Migrans. Acta Derm. Venereol. 2004;84:106–110. doi: 10.1080/00015550310006815. [DOI] [PubMed] [Google Scholar]
- 454.Di Renzi S., Martini A., Binazzi A., Marinaccio A., Vonesch N., D’amico W., Moro T., Fiorentini C., Ciufolini M.G., Visca P., et al. Risk of acquiring tick-borne infections in forestry workers from Lazio, Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:1579–1581. doi: 10.1007/s10096-010-1028-6. [DOI] [PubMed] [Google Scholar]
- 455.Stufano A., Iatta R., Sgroi G., Jahantigh H.R., Cagnazzo F., Floel A., Lucchese G., Loconsole D., Centrone F., Mendoza-Roldan J.A., et al. Seroprevalence of vector-borne pathogens in outdoor workers from southern Italy and associated occupational risk factors. Parasites Vectors. 2022;15:264. doi: 10.1186/s13071-022-05385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Stinco G., Ruscio M., Bergamo S., Trotter D., Patrone P. Clinical features of 705 Borrelia burgdorferi seropositive patients in an endemic area of northern Italy. Sci. World J. 2014;2014:414505. doi: 10.1155/2014/414505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Santino I., Cammarata E., Franco S., Galdiero F., Oliva B., Sessa R., Cipriani P., Tempera G., Del Piano M. Multicentric study of seroprevalence of Borrelia burgdorferi and Anaplasma phagocytophila in high-risk groups in regions of central and southern Italy. Int. J. Immunopathol. Pharmacol. 2004;17:219–223. doi: 10.1177/039463200401700214. [DOI] [PubMed] [Google Scholar]
- 458.Santino I., Dastoli F., Lavorino C., Navazio M., Nicosia R., Oliveti A., Oliveti G., Pustorino R., Sessa R., Del Piano M. Deter-mination of antibodies to Borrelia burgdorferi in the serum of patients living in Calabria, southern Italy. Panminerva Med. 1996;38:167–172. [PubMed] [Google Scholar]
- 459.Cimmino M.A., Azzolini A., Tobia F., Pesce C.M. Spirochetes in the spleen of a patient with chronic Lyme disease. Am. J. Clin. Pathol. 1989;91:95–97. doi: 10.1093/ajcp/91.1.95. [DOI] [PubMed] [Google Scholar]
- 460.Beltrame A., Rodari P., Mauroner L., Zanella F., Moro L., Bertoli G., Da Re F., Russo F., Napoletano G., Silva R. Emergence of Lyme borreliosis in the province of Verona, Northern Italy: Five-years of sentinel surveillance. Ticks Tick Borne Dis. 2021;12:101628. doi: 10.1016/j.ttbdis.2020.101628. [DOI] [PubMed] [Google Scholar]
- 461.Pugliese A., Beltramo T., Torre D. Seroprevalence study of Tick-borne encephalitis, Borrelia burgdorferi, Dengue and Toscana virus in Turin Province. Cell Biochem. Funct. 2007;25:185–188. doi: 10.1002/cbf.1302. [DOI] [PubMed] [Google Scholar]
- 462.Santino I., Sessa R., Di Pietro M., Del Piano M. Lyme borreliosis in central Italy (1995–1998) New Microbiol. 2000;23:261–269. [PubMed] [Google Scholar]
- 463.Ciceroni L., Bartoloni A., Leoncini F., Ciarrocchi S., Pinto A., Favia G., Bartalesi F., Scagnoli L., Iori A. Risk of tick-borne bacterial diseases in humans in the Florence area, Tuscany. Ann. N. Y. Acad. Sci. 2003;990:346–349. doi: 10.1111/j.1749-6632.2003.tb07386.x. [DOI] [PubMed] [Google Scholar]
- 464.Fumarola D., Marcuccio C., Crovato F., Nazzari G., Rovetta G., Cimmino M.A., Bianchi G. Lyme disease in Italy: First reported case. Boll Ist Sieroter Milan. 1985;64:483–485. [PubMed] [Google Scholar]
- 465.Milanese R., Marin M., Antonini-Canterin A., Crovatto M., Modolo M.L., Scian I., Cilia A.M., Santini G.F. Borreliosis risk after tick bite in north-eastern Italy. Microbiologica. 1991;14:257–259. [PubMed] [Google Scholar]
- 466.Trevisan G., Ruscio M., Cinco M., Nan K., Forgione P., Di Meo N., Tranchini P., Nacca M., Trincone S., Rimoldi S.G., et al. The history of Lyme disease in Italy and its spread in the Italian territory. Front. Pharmacol. 2023;14:1128142. doi: 10.3389/fphar.2023.1128142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Cinco M., De Giovannini R. Protein and antigenic analysis of Borrelia burgdorferi isolated in northern Italy: Computerized analysis of phenotypic characteristics. J. Clin. Microbiol. 1993;31:440–443. doi: 10.1128/jcm.31.2.440-443.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Cinco M., Murgia R., Ruscio M., Andriolo B. IgM and IgG significant reactivity to Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii among Italian patients affected by Lyme arthritis or neuroborreliosis. FEMS Immunol. Med. Microbiol. 1996;14:159–166. doi: 10.1111/j.1574-695x.1996.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 469.Tomasini C. Cordoniform morphea: A clinicopathologic study of two cases presenting with the rope sign. J. Cutan. Pathol. 2016;43:613–622. doi: 10.1111/cup.12704. [DOI] [PubMed] [Google Scholar]
- 470.Cinco M., Murgia R., Costantini C. Prevalence of IgG reactivity in Lyme borreliosis patients versus Borrelia garinii and Borrelia afzelii in a restricted area of Northern Italy. FEMS Immunol. Med Microbiol. 1995;12:217–222. doi: 10.1111/j.1574-695x.1995.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 471.Matera G., Labate A., Quirino A., Lamberti A.G., Borz A.G., Barreca G.S., Mumoli L., Peronace C., Giancotti A., Gambardella A., et al. Chronic neuroborreliosis by B. garinii: An unusual case presenting with epilepsy and multifocal brain MRI lesions. New Microbiol. 2014;37:393–397. [PubMed] [Google Scholar]
- 472.Ruzic-Sabljic E., Maraspin V., Lotric-Furlan S., Jurca T., Logar M., Pikelj-Pecnik A., Strle F. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin Wochenschr. 2002;114:544–550. [PubMed] [Google Scholar]
- 473.Picken R.N., Cheng Y., Strle F., Cimperman J., Maraspin V., Lotric-Furlan S., Ruzic-Sabljic E., Han D., Nelson J.A., Picken M.M., et al. Molecular characterization of Borrelia burgdorferi sensu lato from Slovenia revealing significant differences between tick and human isolates. Eur. J. Clin. Microbiol. Infect. Dis. 1996;15:313–323. doi: 10.1007/bf01695664. [DOI] [PubMed] [Google Scholar]
- 474.Ruzic-Sabljic E., Strle F., Cimperman J., Maraspin V., Lotric-Furlan S., Pleterski-Rigler D. Characterisation of Borrelia burgdorferi sensu lato strains isolated from patients with skin manifestations of Lyme borreliosis residing in Slovenia. J. Med. Microbiol. 2000;49:47–53. doi: 10.1099/0022-1317-49-1-47. [DOI] [PubMed] [Google Scholar]
- 475.Strle F., Ruzic-Sabljic E., Cimperman J., Lotric-Furlan S., Maraspin V. Comparison of Findings for Patients with Borrelia garinii and Borrelia afzelii Isolated from Cerebrospinal Fluid. Clin. Infect. Dis. 2006;43:704–710. doi: 10.1086/506936. [DOI] [PubMed] [Google Scholar]
- 476.Cizman M., Avsic-Zupanc T., Petrovec M., Ruzic-Sabljic E., Pokorn M. Seroprevalence of ehrlichiosis, Lyme borreliosis and tick-borne encephalitis infections in children and young adults in Slovenia. Wien Klin Wochenschr. 2000;112:842–845. [PubMed] [Google Scholar]
- 477.Logar M., Ruzic-Sabljic E., Maraspin V., Lotric-Furlan S., Cimperman J., Jurca T., Strle F. Comparison of Erythema Migrans Caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32:15–19. doi: 10.1007/s15010-004-3042-z. [DOI] [PubMed] [Google Scholar]
- 478.Strle F., Nadelman R.B., Cimperman J., Nowakowski J., Picken R.N., Schwartz I., Maraspin V., Aguero-Rosenfeld M.E., Varde S., Lotric-Furlan S., et al. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann. Intern. Med. 1999;130:32–36. doi: 10.7326/0003-4819-130-1-199901050-00006. [DOI] [PubMed] [Google Scholar]
- 479.Rojko T., Bogovic P., Lotric-Furlan S., Ogrinc K., Cerar-Kisek T., Glinsek Biskup U., Petrovec M., Ruzic-Sabljic E., Kastrin A., Strle F. Borrelia burgdorferi sensu lato infection in patients with peripheral facial palsy. Ticks Tick Borne Dis. 2019;10:398–406. doi: 10.1016/j.ttbdis.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 480.Arnez M., Ruzic-Sabljic E. Borrelia burgdorferi Sensu Lato Bacteremia in Slovenian Children with Solitary and Multiple Erythema Migrans. Pediatr. Infect. Dis. J. 2011;30:988–990. doi: 10.1097/inf.0b013e318225b8c3. [DOI] [PubMed] [Google Scholar]
- 481.Rozic M., Lah L.L., Ruzic-Sabljic E., Kastrin A., Arnez M. Lyme Neuroborreliosis in Children: Etiology and Comparison of Clinical Findings of Lyme Neuroborreliosis Caused by Borrelia garinii and Borrelia afzelii. Pediatr. Infect. Dis. J. 2019;38:E279–E284. doi: 10.1097/INF.0000000000002415. [DOI] [PubMed] [Google Scholar]
- 482.Stupica D., Lusa L., Maraspin V., Bogovic P., Vidmar D., O’rourke M., Traweger A., Livey I., Strle F. Correlation of Culture Positivity, PCR Positivity, and Burden of Borrelia burgdorferi Sensu Lato in Skin Samples of Erythema Migrans Patients with Clinical Findings. PLoS ONE. 2015;10:e0136600. doi: 10.1371/journal.pone.0136600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Cerar T., Ruzic-Sabljic E., Glinsek U., Zore A., Strle F. Comparison of PCR methods and culture for the detection of Borrelia spp. in patients with erythema migrans. Clin. Microbiol. Infect. 2008;14:653–658. doi: 10.1111/j.1469-0691.2008.02013.x. [DOI] [PubMed] [Google Scholar]
- 484.Maraspin V., Ruzic-Sabljic E., Cimperman J., Lotric-Furlan S., Jurca T., Picken R.N., Strle F. Isolation of Borrelia burgdorferi Sensu Lato from Blood of Patients with Erythema Migrans. Infection. 2001;29:65–70. doi: 10.1007/s15010-001-0154-6. [DOI] [PubMed] [Google Scholar]
- 485.Maraspin V., Ogrinc K., Rojko T., Bogovic P., Ruzic-Sabljic E., Kastrin A., Wormser G.P., Strle F. Characteristics of spirochetemic patients with a solitary erythema migrans skin lesion in Europe. PLoS ONE. 2021;16:e0250198. doi: 10.1371/journal.pone.0250198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Ruzic-Sabljic E., Arnez M., Logar M., Maraspin V., Lotric-Furlan S., Cimperman J., Strle F. Comparison of Borrelia burgdorferi sensu lato strains isolated from specimens obtained simultaneously from two different sites of infection in individual patients. J. Clin. Microbiol. 2005;43:2194–2200. doi: 10.1128/jcm.43.5.2194-2200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Ruzic-Sabljic E., Arnez M., Lotric-Furlan S., Maraspin V., Cimperman J., Strle F. Genotypic and phenotypic characterisation of Borrelia burgdorferi sensu lato strains isolated from human blood. J. Med. Microbiol. 2001;50:896–901. doi: 10.1099/0022-1317-50-10-896. [DOI] [PubMed] [Google Scholar]
- 488.Picken R.N., Strle F., Ruzic-Sabljic E., Maraspin V., Lotric-Furlan S., Cimperman J., Cheng Y., Picken M.M. Molecular subtyping of Borrelia burgdorferi sensu lato isolates from five patients with solitary lymphocytoma. J. Investig. Dermatol. 1997;108:92–97. doi: 10.1111/1523-1747.ep12285646. [DOI] [PubMed] [Google Scholar]
- 489.Arnez M., Pleterski-Rigler D., Luznik-Bufon T., Ruzic-Sabljic E., Strle F. Solitary and multiple erythema migrans in children: Comparison of demographic, clinical and laboratory findings. Infection. 2003;31:404–409. doi: 10.1007/s15010-003-4007-3. [DOI] [PubMed] [Google Scholar]
- 490.Zore A., Ruzic-Sabljic E., Maraspin V., Cimperman J., Lotric-Furlan S., Pikelj A., Jurca T., Logar M., Strle F. Sensitivity of culture and polymerase chain reaction for the etiologic diagnosis of erythema migrans. Wien Klin Wochenschr. 2002;114:606–609. [PubMed] [Google Scholar]
- 491.Strle F., Ruzic-Sabljic E., Logar M., Maraspin V., Lotrič-Furlan S., Cimperman J., Ogrinc K., Stupica D., Nadelman R.B., Nowakowski J., et al. Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector-Borne Zoonotic Dis. 2011;11:1253–1258. doi: 10.1089/vbz.2010.0230. [DOI] [PubMed] [Google Scholar]
- 492.Ogrinc K., Lusa L., Lotric-Furlan S., Bogovic P., Stupica D., Cerar T., Ruzic-Sabljic E., Strle F. Course and Outcome of Early European Lyme Neuroborreliosis (Bannwarth Syndrome): Clinical and Laboratory Findings. Clin. Infect. Dis. 2016;63:346–353. doi: 10.1093/cid/ciw299. [DOI] [PubMed] [Google Scholar]
- 493.Becker N.S., Rollins R.E., Nosenko K., Paulus A., Martin S., Krebs S., Takano A., Sato K., Kovalev S.Y., Kawabata H., et al. High conservation combined with high plasticity: Genomics and evolution of Borrelia bavariensis. BMC Genom. 2020;21:702. doi: 10.1186/s12864-020-07054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Strle F., Picken R.N., Cheng Y., Cimperman J., Maraspin V., Lotric-Furlan S., Ruzic-Sabljic E., Picken M.M. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin. Infect. Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]
- 495.Tijsse-Klasen E., Sprong H., Pandak N. Co-infection of Borrelia burgdorferi sensu lato and Rickettsia species in ticks and in an erythema migrans patient. Parasites Vectors. 2013;6:347. doi: 10.1186/1756-3305-6-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Poljak I., Troselj-Vukic B., Miletic B., Morovic M., Ruzic-Sabljic E., Vucemilovic A., Materljan E. Low sero-prevalence of Lyme borreliosis in the forested mountainous area of Gorski Kotar, Croatia. Croat. Med. J. 2000;41:433–436. [PubMed] [Google Scholar]
- 497.Habek M., Mubrin Z., Brinar V.V. Avellis syndrome due to borreliosis. Eur. J. Neurol. 2007;14:112–114. doi: 10.1111/j.1468-1331.2006.01528.x. [DOI] [PubMed] [Google Scholar]
- 498.Topolovec J., Puntaric D., Antolovic-Pozgain A., Vukovic D., Topolovec Z., Milas J., Drusko-Barisic V., Venus M. Serologically detected “new” tick-borne zoonoses in eastern Croatia. Croat. Med. J. 2003;44:626–629. [PubMed] [Google Scholar]
- 499.Juric S., Janculjak D., Tomic S., Butkovic Soldo S., Bilic E. Epileptic seizure as initial and only manifestation of neuroborreliosis: Case report. Neurol. Sci. 2014;35:793–794. doi: 10.1007/s10072-014-1648-1. [DOI] [PubMed] [Google Scholar]
- 500.Vukelic D., Bozinovic D., Morovic M., Tesovic G., Ruzic Sabljic E., Barisic N., Knezovic I. Opsoclonus-myoclonus syndrome in a child with neuroborreliosis. J. Infect. 2000;40:189–191. doi: 10.1016/s0163-4453(00)80016-x. [DOI] [PubMed] [Google Scholar]
- 501.Golubic D., Zember S. Dual infection: Tularemia and Lyme borreliosis acquired by single tick bite in northwest Croatia. Acta Med. Croatica. 2001;55:207–209. [PubMed] [Google Scholar]
- 502.Golubic D., Rijpkema S., Tkalec-Makovec N., Ruzic E. Epidemiologic, ecologic and clinical characteristics of Lyme borrel-liosis in northwest Croatia. Acta Med. Croatica. 1998;52:7–13. [PubMed] [Google Scholar]
- 503.Blazina K., Martinez I., Foro Znika M. Bilateral facial nerve palsy as a presentation of coexisting neuroborreliosis and post-acute COVID-19 syndrome. Croat. Med. J. 2023;64:440–443. doi: 10.3325/cmj.2023.64.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Situm M., Poje G., Grahovac B., Marinovic B., Levanat S. Diagnosis of lyme borreliosis by polymerase chain reaction. Clin. Dermatol. 2002;20:147–155. doi: 10.1016/s0738-081x(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 505.Bucak O., Kocoglu M.E., Tas T., Mengeloglu F.Z. Evaluation of Borrelia burgdorferi sensu lato seroprevalence in the province of Bolu, Turkey. Turk. J. Med. Sci. 2016;46:727–732. doi: 10.3906/sag-1504-100. [DOI] [PubMed] [Google Scholar]
- 506.Akar N., Caliskan E., Ozturk C.E., Ankarali H., Kilincel O., Oksuz S., Sahin I. Seroprevalence of hantavirus and Borreliaburgdorferi in Düzce (Turkey) forest villages and the relationship with sociodemographic features. Turk. J. Med. Sci. 2019;49:483–489. doi: 10.3906/sag-1807-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Cikman A., Aydin M., Gulhan B., Karakecili F., Demirtas L., Kesik O.A. Geographical Features and Seroprevalence of Bor-relia burgdorferi in Erzincan, Turkey. J. Arthropod Borne Dis. 2018;12:378–386. [PMC free article] [PubMed] [Google Scholar]
- 508.Cora M., Kaklıkkaya N., Topbas M., Can G., Yavuzyilmaz A., Tosun I., Aydın F. Determination of Seroprevalence of Borrelia burgdorferi IgG in Adult Population Living in Trabzon. Balk. Med. J. 2017;34:47–52. doi: 10.4274/balkanmedj.2015.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Onen F., Tuncer D., Akar S., Birlik M., Akkoc N. Seroprevalence of Borrelia burgdorferi in patients with Behcet’s disease. Rheumatol. Int. 2003;23:289–293. doi: 10.1007/s00296-003-0313-4. [DOI] [PubMed] [Google Scholar]
- 510.Ozcan Y., Gunes Takir S., Karagun E., Uyar B. Dermatoscopic Features of Early Erythema Chronicum Migrans. Acta Dermatovenerol. Croat. 2023;31:110–112. [PubMed] [Google Scholar]
- 511.Kouroupis D., Terzaki M., Moscha N., Sarvani A., Simoulidou E., Chatzimichailidou S., Giza E., Sapouridis G., Angelakis E., Petidis K., et al. Aseptic Meningitis Linked to Borrelia afzelii Seroconversion in Northeastern Greece: An Emerging Infectious Disease Contested in the Region. Trop. Med. Infect. Dis. 2024;9:25. doi: 10.3390/tropicalmed9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Chatzipanagiotou S., Papandreou-Rakitzis P., Malamou-Ladas H., Antoniou P. Determination of antibody titres forBorrelia burgdorferi in the serum of gipsies living in Attika, Greece. Eur. J. Clin. Microbiol. Infect. Dis. 1992;11:477–478. doi: 10.1007/bf01961871. [DOI] [PubMed] [Google Scholar]
- 513.Antoniou M., Tselentis Y., Babalis T., Gikas A., Stratigakis N., Vlachonikolis I., Kafatos A., Fioretos M. The seroprevalence of ten zoonoses in two villages of Crete, Greece. Eur. J. Epidemiol. 1995;11:415–423. doi: 10.1007/bf01721226. [DOI] [PubMed] [Google Scholar]
- 514.Stamouli M., Totos G., Braun H.-B., Michel G., Gizaris V. Very low seroprevalence of Lyme borreliosis in young Greek males. Eur. J. Epidemiol. 2000;16:495–496. doi: 10.1023/a:1007657430880. [DOI] [PubMed] [Google Scholar]
- 515.Kalogeropoulos D., Asproudis I., Stefaniotou M., Moschos M., Gartzonika C., Bassukas I., Konitsiotis S., Milionis H., Gaitanis G., Malamos K., et al. Spirochetal uveitis: Spectrum of clinical manifestations, diagnostic and therapeutic approach, final outcome and epidemiological data. Int. Ophthalmol. 2021;41:4111–4126. doi: 10.1007/s10792-021-01984-x. [DOI] [PubMed] [Google Scholar]
- 516.Holubar K. Lyme disease in Israel. J. Am. Acad. Dermatol. 1992;27:486–487. doi: 10.1016/s0190-9622(08)80893-6. [DOI] [PubMed] [Google Scholar]
- 517.Haberberger R.L., Jr., Constantine N.T., Schwan T.G., Woody J.N. Lyme disease agent in Egypt? Trans. R. Soc. Trop. Med. Hyg. 1989;83:556. doi: 10.1016/0035-9203(89)90293-9. [DOI] [PubMed] [Google Scholar]
- 518.Gil H., Barral M., Escudero R., Garcia-Perez A.L., Anda P. Identification of a new Borrelia species among small mammals in areas of northern Spain where Lyme disease is endemic. Appl. Environ. Microbiol. 2005;71:1336–1345. doi: 10.1128/aem.71.3.1336-1345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Solano-Gallego L., Llull J., Osso M., Hegarty B., Breitschwerdt E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet. Res. 2006;37:231–244. doi: 10.1051/vetres:2005054. [DOI] [PubMed] [Google Scholar]
- 520.Pato F.J., Panadero R., Vazquez L., Lopez C.M., Diaz P., Vazquez E., Diez-Baños P., Morrondo P., Fernandez G. Seroprevalence of Borrelia burgdorferi sensu lato in roe deer (Capreolus capreolus) from northwestern Spain. J. Zoo Wildl. Med. 2013;44:660–665. doi: 10.1638/2012-0240r2.1. [DOI] [PubMed] [Google Scholar]
- 521.Barandika J.F., Hurtado A., Garcia-Esteban C., Gil H., Escudero R., Barral M., Jado I., Juste R.A., Anda P., Garcia-Perez A.L. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl. Environ. Microbiol. 2007;73:6166–6171. doi: 10.1128/aem.00590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Perez G., Bastian S., Chastagner A., Agoulon A., Plantard O., Vourc’H G., Butet A. Ecological factors influencing small mammal infection by Anaplasma phagocytophilum and Borrelia burgdorferi s.l. in agricultural and forest landscapes. Environ. Microbiol. 2017;19:4205–4219. doi: 10.1111/1462-2920.13885. [DOI] [PubMed] [Google Scholar]
- 523.Deruaz D., Eid P., Deruaz J., Sempere A., Bourgouin C., Rodhain F., Perez-Eid C. Use of enzyme-labelled protein G assay for the detection of anti Borrelia burgdorferi antibodies in wild animal sera. Eur. J. Epidemiol. 1996;12:515–519. doi: 10.1007/bf00144006. [DOI] [PubMed] [Google Scholar]
- 524.Ollivier V., Choquet R., Gamble A., Bastien M., Combes B., Gilot-Fromont E., Pellerin M., Gaillard J.M., Lemaitre J.F., Verheyden H., et al. Temporal dynamics of antibody level against Lyme disease bacteria in roe deer: Tale of a sentinel? Ecol. Evol. 2023;13:e10414. doi: 10.1002/ece3.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Lebert I., Agoulon A., Bastian S., Butet A., Cargnelutti B., Cebe N., Chastagner A., Léger E., Lourtet B., Masseglia S., et al. Distribution of ticks, tick-borne pathogens and the associated local environmental factors including small mammals and livestock, in two French agricultural sites: The OSCAR database. Biodivers. Data J. 2020;8:e50123. doi: 10.3897/bdj.8.e50123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Buffet J.-P., Marsot M., Vaumourin E., Gasqui P., Masseglia S., Marcheteau E., Huet D., Chapuis J.-L., Pisanu B., Ferquel E., et al. Co-infection of Borrelia afzelii and Bartonella spp. in bank voles from a suburban forest. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:583–589. doi: 10.1016/j.cimid.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 527.Traversa D., Milillo P., Maggi R., Simonato G., Di Cesare A., Pezzuto C., Grillini M., Morelli S., Colombo M., Passarelli A., et al. Seroexposure to Zoonotic Anaplasma and Borrelia in Dogs and Horses That Are in Contact with Vulnerable People in Italy. Pathogens. 2023;12:470. doi: 10.3390/pathogens12030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Petruccelli A., Ferrara G., Iovane G., Schettini R., Ciarcia R., Caputo V., Pompameo M., Pagnini U., Montagnaro S. Seroprevalence of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi sensu lato, and Dirofilaria immitis in Stray Dogs, from 2016 to 2019, in Southern Italy. Animals. 2020;11:9. doi: 10.3390/ani11010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Laus F., Veronesi F., Passamonti F., Paggi E., Cerquetella M., Hyatt D., Tesei B., Fioretti D.P. Prevalence of Tick Borne Pathogens in Horses from Italy. J. Vet. Med Sci. 2013;75:715–720. doi: 10.1292/jvms.12-0449. [DOI] [PubMed] [Google Scholar]
- 530.Mendoza-Roldan J.A., Benelli G., Bezerra-Santos M.A., Nguyen V.L., Conte G., Iatta R., Furlanello T., Otranto D. Seropositivity to canine tick-borne pathogens in a population of sick dogs in Italy. Parasites Vectors. 2021;14:292. doi: 10.1186/s13071-021-04772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Galluzzo P., Grippi F., Di Bella S., Santangelo F., Sciortino S., Castiglia A., Sciacca C., Arnone M., Alduina R., Chiarenza G. Seroprevalence of Borrelia burgdorferi in Stray Dogs from Southern Italy. Microorganisms. 2020;8:1688. doi: 10.3390/microorganisms8111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Morelli S., Gori F., Colombo M., Traversa D., Sarrocco G., Simonato G., Nespeca C., Di Cesare A., Frangipane di Regalbono A., Veronesi F., et al. Simultaneous Exposure to Angiostrongylus vasorum and Vector-Borne Pathogens in Dogs from Italy. Pathogens. 2021;10:1200. doi: 10.3390/pathogens10091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Colombo M., Morelli S., Simonato G., Di Cesare A., Veronesi F., Frangipane di Regalbono A., Grassi L., Russi I., Tiscar P.G., Morganti G., et al. Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy. Pathogens. 2021;10:507. doi: 10.3390/pathogens10050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Giudice E., Domina F., Britt D., Di Pietro S., Pugliese A. Clinical findings associated with Borrelia burgdorferi infection in the dog. Vet. Res. Commun. 2003;27((Suppl. 1)):767–770. doi: 10.1023/b:verc.0000014267.25428.32. [DOI] [PubMed] [Google Scholar]
- 535.Ebani V.V., Guardone L., Rocchigiani G., Bascherini A., Cagnoli G., Bertelloni F., Bongi P., Russo C., Riccioli F., Mancianti F. Molecular survey on the presence of arthropod-borne bacteria and protozoans in roe deer (Capreolus capreolus) and ticks from Central Italy. Acta Trop. 2022;233:106586. doi: 10.1016/j.actatropica.2022.106586. [DOI] [PubMed] [Google Scholar]
- 536.Ciceroni L., Simeoni J., Pacetti A.I., Ciarrocchi S., Cacciapuoti B. Antibodies to Borrelia burgdorferi in sheep and goats. Alto Adige-South Tyrol, Italy. New Microbiol. 1996;19:171–174. [PubMed] [Google Scholar]
- 537.Zore A., Petrovec M., Prosenc K., Trilar T., Ruzic-Sabljic E., Avsic-Zupanc T. Infection of small mammals with Borrelia burgdorferi sensu lato in Slovenia as determined by polymerase chain reaction (PCR) Wien Klin Wochenschr. 1999;111:997–999. [PubMed] [Google Scholar]
- 538.Athanasiou L.V., Tsokana C.N., Gougoulis D.A., Tzivara A.H., Dedousi A., Katsoulos P.D. Natural Co-Exposure to Borrelia burgdorferi s.l. and Anaplasma phagocytophilum: Unraveling the Hematological Profile in Sheep. Life. 2023;13:469. doi: 10.3390/life13020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Ortega N., Arcenillas-Hernandez I., Villa M., Gonzalez M.D., Caro M.R. Molecular identification of Borrelia and SFG Rickettsia spp. in hard ticks parasitizing domestic and wild animals in southeastern Spain. Vet. Res. Commun. 2024;48:1785–1790. doi: 10.1007/s11259-023-10292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Palomar A.M., Portillo A., Santibanez P., Santibanez S., Oteo J.A. Borrelia miyamotoi: Should this pathogen be considered for the diagnosis of tick-borne infectious diseases in Spain? Enfermedades Infecc. Microbiol. Clin. 2018;36:568–571. doi: 10.1016/j.eimc.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 541.Estrada-Pena A., Oteo J.A., Estrada-Pena R., Gortazar C., Osacar J.J., Moreno J.A., Castella J. Borrelia burgdorferi sensu lato in ticks (Acari: Ixodidae) from two different foci in Spain. Exp. Appl. Acarol. 1995;19:173–180. doi: 10.1007/bf00046289. [DOI] [PubMed] [Google Scholar]
- 542.Estrada-Pena A., Roura X., Sainz A., Miro G., Solano-Gallego L. Species of ticks and carried pathogens in owned dogs in Spain: Results of a one-year national survey. Ticks Tick Borne Dis. 2017;8:443–452. doi: 10.1016/j.ttbdis.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 543.Barral M., Garcia-Perez A.L., Juste R.A., Hurtado A., Escudero R., Sellek R.E., Anda P. Distribution of Borrelia burgdorferi sensu lato in Ixodes ricinus (Acari: Ixodidae) Ticks from the Basque Country, Spain. J. Med. Ѐntomol. 2002;39:177–184. doi: 10.1603/0022-2585-39.1.177. [DOI] [PubMed] [Google Scholar]
- 544.Barandika J.F., Hurtado A., Garcia-Sanmartin J., Juste R.A., Anda P., Garcia-Perez A.L. Prevalence of tick-borne zoonotic bacteria in questing adult ticks from northern Spain. Vector Borne Zoonotic Dis. 2008;8:829–835. doi: 10.1089/vbz.2008.0023. [DOI] [PubMed] [Google Scholar]
- 545.Toledo A., Olmeda A.S., Escudero R., Jado I., Valcarcel F., Casado-Nistal M.A., Rodriguez-Vargas M., Gil H., Anda P. Tick-borne zoonotic bacteria in ticks collected from central Spain. Am. J. Trop. Med. Hyg. 2009;81:67–74. doi: 10.4269/ajtmh.2009.81.67. [DOI] [PubMed] [Google Scholar]
- 546.Delgado J.D., Abreu-Yanes E., Abreu-Acosta N., Flor M.D., Foronda P. Vertebrate Ticks Distribution and Their Role as Vectors in Relation to Road Edges and Underpasses. Vector Borne Zoonotic Dis. 2017;17:376–383. doi: 10.1089/vbz.2016.2073. [DOI] [PubMed] [Google Scholar]
- 547.Palomar A.M., Santibanez P., Mazuelas D., Roncero L., Santibanez S., Portillo A., Oteo J.A. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg. Infect. Dis. 2012;18:1188–1191. doi: 10.3201/eid1807.111777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Merino F.J., Nebreda T., Serrano J.L., Fernandez-Soto P., Encinas A., Perez-Sanchez R. Tick species and tick-borne infections identified in population from a rural area of Spain. Epidemiol. Infect. 2005;133:943–949. doi: 10.1017/s0950268805004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Estrada-Pena A., Osacar J.J., Pichon B., Gray J.S. Hosts and pathogen detection for immature stages of Ixodes ricinus (Acari: Ixodidae) in North-Central Spain. Exp. Appl. Acarol. 2005;37:257–268. doi: 10.1007/s10493-005-3271-6. [DOI] [PubMed] [Google Scholar]
- 550.Ruiz-Fons F., Fernandez-De-Mera I.G., Acevedo P., Gortazar C., de la Fuente J. Factors driving the abundance of ixodes ricinus ticks and the prevalence of zoonotic I. ricinus-borne pathogens in natural foci. Appl. Environ. Microbiol. 2012;78:2669–2676. doi: 10.1128/aem.06564-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Nebbak A., Dahmana H., Almeras L., Raoult D., Boulanger N., Jaulhac B., Mediannikov O., Parola P. Co-infection of bacteria and protozoan parasites in Ixodes ricinus nymphs collected in the Alsace region, France. Ticks Tick Borne Dis. 2019;10:101241. doi: 10.1016/j.ttbdis.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 552.Boyer P.H., Boulanger N., Nebbak A., Collin E., Jaulhac B., Almeras L. Assessment of MALDI-TOF MS biotyping for Borrelia burgdorferi sl detection in Ixodes ricinus. PLoS ONE. 2017;12:e0185430. doi: 10.1371/journal.pone.0185430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Geurden T., Becskei C., Six R.H., Maeder S., Latrofa M.S., Otranto D., Farkas R. Detection of tick-borne pathogens in ticks from dogs and cats in different European countries. Ticks Tick Borne Dis. 2018;9:1431–1436. doi: 10.1016/j.ttbdis.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 554.Michelet L., Joncour G., Devillers E., Torina A., Vayssier-Taussat M., Bonnet S.I., Moutailler S. Tick species, tick-borne pathogens and symbionts in an insular environment off the coast of Western France. Ticks Tick Borne Dis. 2016;7:1109–1115. doi: 10.1016/j.ttbdis.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 555.Socolovschi C., Reynaud P., Kernif T., Raoult D., Parola P. Rickettsiae of spotted fever group, Borrelia valaisiana, and Coxiella burnetii in ticks on passerine birds and mammals from the Camargue in the south of France. Ticks Tick Borne Dis. 2012;3:355–360. doi: 10.1016/j.ttbdis.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 556.Halos L., Jamal T., Maillard R., Beugnet F., Le Menach A., Boulouis H.J., Vayssier-Taussat M. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet. Res. 2005;36:79–87. doi: 10.1051/vetres:2004052. [DOI] [PubMed] [Google Scholar]
- 557.Halos L., Vourc’h G., Cotte V., Gasqui P., Barnouin J., Boulous H.J., Vayssier-Taussat M. Prevalence of Anaplasma phagocytophilum, Rickettsia sp. and Borrelia burgdorferi sensu lato DNA in Questing Ixodes ricinus Ticks from France. Ann. N. Y. Acad. Sci. 2006;1078:316–319. doi: 10.1196/annals.1374.059. [DOI] [PubMed] [Google Scholar]
- 558.Ferquel E., Garnier M., Marie J., Bernède-Bauduin C., Baranton G., Perez-Eid C., Postic D. Prevalence of Borrelia burgdorferi sensu lato and Anaplasmataceae members in Ixodes ricinus ticks in Alsace, a focus of Lyme borreliosis endemicity in France. Appl. Environ. Microbiol. 2006;72:3074–3078. doi: 10.1128/aem.72.4.3074-3078.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Vayssier-Taussat M., Moutailler S., Michelet L., Devillers E., Bonnet S., Cheval J., Hebert C., Eloit M. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS ONE. 2013;8:e81439. doi: 10.1371/journal.pone.0081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Quessada T., Martial-Convert F., Arnaud S., Leudet De La Vallee H., Gilot B., Pichot J. Prevalence of Borrelia burgdorferi species and identification of Borrelia valaisiana in questing Ixodes ricinus in the Lyon region of France as determined by polymerase chain reaction-restriction fragment length polymorphism. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22:165–173. doi: 10.1007/s10096-002-0866-2. [DOI] [PubMed] [Google Scholar]
- 561.Gilot B., Degeilh B., Pichot J., Doche B., Guiguen C. Prevalence of Borrelia burgdorferi (sensu lato) in Ixodes ricinus (L.) populations in France, according to a phytoecological zoning of the territory. Eur. J. Epidemiol. 1996;12:395–401. doi: 10.1007/bf00145304. [DOI] [PubMed] [Google Scholar]
- 562.Reis C., Cote M., Paul R.E., Bonnet S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector-Borne Zoonotic Dis. 2011;11:907–916. doi: 10.1089/vbz.2010.0103. [DOI] [PubMed] [Google Scholar]
- 563.Moutailler S., Valiente Moro C., Vaumourin E., Michelet L., Tran F.H., Devillers E., Cosson J.F., Gasqui P., Van V.T., Mavingui P., et al. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl. Trop. Dis. 2016;10:e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Zhioua E., Postic D., Rodhain F., Perez-Eid C. Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi in Ile de France. J. Med. Ѐntomol. 1996;33:694–697. doi: 10.1093/jmedent/33.4.694. [DOI] [PubMed] [Google Scholar]
- 565.Beytout J., George J.C., Malaval J., Garnier M., Beytout M., Baranton G., Ferquel E., Postic D. Lyme borreliosis incidence in two french departments: Correlation with infection of Ixodes ricinus ticks by Borrelia burgdorferi sensu lato. Vector Borne Zoonotic Dis. 2007;7:507–517. doi: 10.1089/vbz.2006.0633. [DOI] [PubMed] [Google Scholar]
- 566.Richter D., Schlee D.B., Matuschka F.R. Relapsing fever-like spirochetes infecting european vector tick of lyme disease agent. Emerg. Infect. Dis. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Vourc’h G., Abrial D., Bord S., Jacquot M., Masseglia S., Poux V., Pisanu B., Bailly X., Chapuis J.L. Mapping human risk of infection with Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in a periurban forest in France. Ticks Tick Borne Dis. 2016;7:644–652. doi: 10.1016/j.ttbdis.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 568.Lejal E., Moutailler S., Simo L., Vayssier-Taussat M., Pollet T. Tick-borne pathogen detection in midgut and salivary glands of adult Ixodes ricinus. Parasites Vectors. 2019;12:152. doi: 10.1186/s13071-019-3418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Akl T., Bourgoin G., Souq M.-L., Appolinaire J., Poirel M.-T., Gibert P., Abi Rizk G., Garel M., Zenner L. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite. 2019;26:20. doi: 10.1051/parasite/2019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Boyer P.H., Barthel C., Mohseni-Zadeh M., Talagrand-Reboul E., Frickert M., Jaulhac B., Boulanger N. Impact of Different Anthropogenic Environments on Ticks and Tick-Associated Pathogens in Alsace, a French Region Highly Endemic for Tick-Borne Diseases. Microorganisms. 2022;10:245. doi: 10.3390/microorganisms10020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Bonnet S.I., Paul R.E.L., Bischoff E., Cote M., Le Naour E. First identification of Rickettsia helvetica in questing ticks from a French Northern Brittany Forest. PLoS Neglected Trop. Dis. 2017;11:e0005416. doi: 10.1371/journal.pntd.0005416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Pichon B., Mousson L., Figureau C., Rodhain F., Perez-Eid C. Density of deer in relation to the prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus nymphs in Rambouillet forest, France. Exp. Appl. Acarol. 1999;23:267–275. doi: 10.1023/a:1006023115617. [DOI] [PubMed] [Google Scholar]
- 573.Jacquot M., Gonnet M., Ferquel E., Abrial D., Claude A., Gasqui P., Choumet V., Charras-Garrido M., Garnier M., Faure B., et al. Comparative population genomics of the Borrelia burgdorferi species complex reveals high degree of genetic isolation among species and underscores benefits and constraints to studying intra-specific epidemiological processes. PLoS ONE. 2014;9:e94384. doi: 10.1371/journal.pone.0094384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Halos L., Bord S., Cotte V., Gasqui P., Abrial D., Barnouin J., Boulouis H.-J., Vayssier-Taussat M., Vourc’h G. Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl. Environ. Microbiol. 2010;76:4413–4420. doi: 10.1128/aem.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Yssouf A., Flaudrops C., Drali R., Kernif T., Socolovschi C., Berenger J.-M., Raoult D., Parola P. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid identification of tick vectors. J. Clin. Microbiol. 2013;51:522–528. doi: 10.1128/jcm.02665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Paul R.E., Cote M., Le Naour E., Bonnet S.I. Environmental factors influencing tick densities over seven years in a French suburban forest. Parasites Vectors. 2016;9:309. doi: 10.1186/s13071-016-1591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Ehrmann S., Ruyts S.C., Scherer-Lorenzen M., Bauhus J., Brunet J., Cousins S.A.O., Deconchat M., Decocq G., De Frenne P., De Smedt P., et al. Habitat properties are key drivers of Borrelia burgdorferi (s.l.) prevalence in Ixodes ricinus populations of deciduous forest fragments. Parasites Vectors. 2018;11:23. doi: 10.1186/s13071-017-2590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Gomez-Diaz E., Boulinier T., Sertour N., Cornet M., Ferquel E., McCoy K.D. Genetic structure of marine Borrelia garinii and population admixture with the terrestrial cycle of Lyme borreliosis. Environ. Microbiol. 2011;13:2453–2467. doi: 10.1111/j.1462-2920.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- 579.Halos L., Mavris M., Vourc’h G., Maillard R., Barnouin J., Boulouis H.J., Vayssier-Taussat M. Broad-range PCR-TTGE for the first-line detection of bacterial pathogen DNA in ticks. Vet. Res. 2006;37:245–253. doi: 10.1051/vetres:2005055. [DOI] [PubMed] [Google Scholar]
- 580.Pichon B., Godfroid E., Hoyois B., Bollen A., Rodhain F., Perez-Eid C. Simultaneous infection of Ixodes ricinus nymphs by two Borrelia burgdorferi sensu lato species: Possible implications for clinical manifestations. Emerg. Infect. Dis. 1995;1:89–90. doi: 10.3201/eid0103.950304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Alafaci A., Crepin A., Beaubert S., Berjeaud J.M., Delafont V., Verdon J. Exploring the Individual Bacterial Microbiota of Questing Ixodes ricinus Nymphs. Microorganisms. 2021;9:1526. doi: 10.3390/microorganisms9071526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Melis S., Batisti Biffignandi G., Olivieri E., Galon C., Vicari N., Prati P., Moutailler S., Sassera D., Castelli M. High-throughput screening of pathogens in Ixodes ricinus removed from hosts in Lombardy, northern Italy. Ticks Tick Borne Dis. 2024;15:102285. doi: 10.1016/j.ttbdis.2023.102285. [DOI] [PubMed] [Google Scholar]
- 583.Cinco M., Padovan D., Murgia R., Poldini L., Frusteri L., van de Pol I., Verbeek-De Kruif N., Rijpkema S., Maroli M. Rate of infection of Ixodes ricinus ticks with Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii and group VS116 in an endemic focus of Lyme disease in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 1998;17:90–94. doi: 10.1007/bf01682162. [DOI] [PubMed] [Google Scholar]
- 584.Beltrame A., Laroche M., Degani M., Perandin F., Bisoffi Z., Raoult D., Parola P. Tick-borne pathogens in removed ticks Veneto, northeastern Italy: A cross-sectional investigation. Travel Med. Infect. Dis. 2018;26:58–61. doi: 10.1016/j.tmaid.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 585.Otranto D., Dantas-Torres F., Giannelli A., Latrofa M.S., Cascio A., Cazzin S., Ravagnan S., Montarsi F., Zanzani S.A., Manfredi M.T., et al. Ticks infesting humans in Italy and associated pathogens. Parasites Vectors. 2014;7:328. doi: 10.1186/1756-3305-7-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Morganti G., Gavaudan S., Canonico C., Ravagnan S., Olivieri E., Diaferia M., Marenzoni M.L., Antognoni M.T., Capelli G., Silaghi C., et al. Molecular Survey on Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi Sensu Lato, and Babesia spp. In Ixodes ricinus Ticks Infesting Dogs in Central Italy. Vector-Borne Zoonotic Dis. 2017;17:743–748. doi: 10.1089/vbz.2017.2154. [DOI] [PubMed] [Google Scholar]
- 587.Nazzi F., Martinelli E., Del Fabbro S., Bernardinelli I., Milani N., Iob A., Pischiutti P., Campello C., D’agaro P. Ticks and Lyme borreliosis in an alpine area in northeast Italy. Med. Vet. Ѐntomol. 2010;24:220–226. doi: 10.1111/j.1365-2915.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- 588.Pintore M.D., Ceballos L., Iulini B., Tomassone L., Pautasso A., Corbellini D., Rizzo F., Mandola M.L., Bardelli M., Peletto S., et al. Detection of Invasive Borrelia burgdorferi Strains in North-Eastern Piedmont, Italy. Zoonoses Public Health. 2015;62:365–374. doi: 10.1111/zph.12156. [DOI] [PubMed] [Google Scholar]
- 589.Zanet S., Battisti E., Pepe P., Ciuca L., Colombo L., Trisciuoglio A., Ferroglio E., Cringoli G., Rinaldi L., Maurelli M.P. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: A country-wide molecular survey. BMC Vet. Res. 2020;16:46. doi: 10.1186/s12917-020-2263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Garcia-Vozmediano A., Giglio G., Ramassa E., Nobili F., Rossi L., Tomassone L. Low Risk Perception about Ticks and Tick-Borne Diseases in an Area Recently Invaded by Ticks in Northwestern Italy. Vet. Sci. 2021;8:131. doi: 10.3390/vetsci8070131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Santino I., Iori A., Nicoletti M., Valletta S., Cimmino C., Scoarughi G., Santapaola D., Sessa R., Del Piano M. Prevalence of Borrelia Burgdorferi sensu lato genomospecies and of the human granulocytic ehrlichiosis (HGE) agent in Ixodes ricinus ticks collected in the area of Monti Lepini, Italy. Int. J. Immunopathol. Pharmacol. 2003;16:105–108. doi: 10.1177/039463200301600203. [DOI] [PubMed] [Google Scholar]
- 592.Ciceroni L., Ciarrocchi S., Simeoni J. Antigenic and genomic analysis of a Borrelia burgdorferi sensu stricto strain isolated from Ixodes ricinus ticks in Alto Adige-South Tyrol, Italy. Eur. J. Epidemiol. 1998;14:511–517. doi: 10.1023/a:1007432408746. [DOI] [PubMed] [Google Scholar]
- 593.Pajoro M., Pistone D., Varotto Boccazzi I., Mereghetti V., Bandi C., Fabbi M., Scattorin F., Sassera D., Montagna M. Molecular screening for bacterial pathogens in ticks (Ixodes ricinus) collected on migratory birds captured in northern Italy. Folia Parasitol. 2018;65 doi: 10.14411/fp.2018.008. [DOI] [PubMed] [Google Scholar]
- 594.Santino I., DEL Piano M., Sessa R., Favia G., Iori A. Detection of four Borrelia burgdorferi genospecies and first report of human granulocytic ehrlichiosis agent in Ixodes ricinus ticks collected in central Italy. Epidemiol. Infect. 2002;129:93–97. doi: 10.1017/s0950268802007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Cinco M., Padovan D., Murgia R., Maroli M., Frusteri L., Heldtander M., E Johansson K.E., Engvall E.O. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J. Clin. Microbiol. 1997;35:3365–3366. doi: 10.1128/jcm.35.12.3365-3366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Pecchioli E., Hauffe H.C., Tagliapietra V., Bandi C., Genchi C., Rizzoli A. Genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from the Autonomous Province of Trento, Italy. Int. J. Med. Microbiol. 2007;297:53–59. doi: 10.1016/j.ijmm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 597.Millet I., Ragionieri M., Tomassone L., Trentin C., Mannelli A. Assessment of the Exposure of People to Questing Ticks Carrying Agents of Zoonoses in Aosta Valley, Italy. Vet. Sci. 2019;6:28. doi: 10.3390/vetsci6010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Pascucci I., Camma C. Lyme disease and the detection of Borrelia burgdorferi genospecies in Ixodes ricinus ticks from central Italy. Vet. Ital. 2010;46:173–188. [PubMed] [Google Scholar]
- 599.Toma L., Mancini F., Di Luca M., Cecere J.G., Bianchi R., Khoury C., Quarchioni E., Manzia F., Rezza G., Ciervo A. Detection of Microbial Agents in Ticks Collected from Migratory Birds in Central Italy. Vector Borne Zoonotic Dis. 2014;14:199–205. doi: 10.1089/vbz.2013.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Aureli S., Galuppi R., Ostanello F., Foley J.E., Bonoli C., Rejmanek D., Rocchi G., Orlandi E., Tampieri M.P. Abundance of questing ticks and molecular evidence for pathogens in ticks in three parks of Emilia-Romagna region of Northern Italy. Ann. Agric. Environ. Med. 2015;22:459–466. doi: 10.5604/12321966.1167714. [DOI] [PubMed] [Google Scholar]
- 601.Cacciapuoti B., Ciceroni L., Ciarrocchi S., Khoury C., Simeoni J. Genetic and phenotypic characterization of Borrelia burgdorferi strains isolated from Ixodes ricinus ticks in the Province of Bolzano, Italy. New Microbiol. 1995;18:169–181. [PubMed] [Google Scholar]
- 602.Ebani V.V., Bertelloni F., Turchi B., Filogari D., Cerri D. Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy. Asian Pac. J. Trop. Med. 2015;8:714–717. doi: 10.1016/j.apjtm.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 603.Favia G., Cancrini G., Carfi A., Grazioli D., Lillini E., Iori A. Molecular identification of Borrelia valaisiana and HGE-like Ehrlichia in Ixodes ricinus ticks sampled in north-eastern Italy: First report in Veneto region. Parassitologia. 2001;43:143–146. [PubMed] [Google Scholar]
- 604.Corrain R., Drigo M., Fenati M., Menandro M.L., Mondin A., Pasotto D., Martini M. Study on Ticks and Tick-Borne Zoonoses in Public Parks in Italy. Zoonoses Public Health. 2012;59:468–476. doi: 10.1111/j.1863-2378.2012.01490.x. [DOI] [PubMed] [Google Scholar]
- 605.Da Rold G., Ravagnan S., Soppelsa F., Porcellato E., Soppelsa M., Obber F., Citterio C.V., Carlin S., Danesi P., Montarsi F., et al. Ticks are more suitable than red foxes for monitoring zoonotic tick-borne pathogens in northeastern Italy. Parasites Vectors. 2018;11:137. doi: 10.1186/s13071-018-2726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Mancini F., Vescio M.F., Toma L., Di Luca M., Severini F., Caccio S.M., Mariano C., Nicolai G., Laghezza Masci V., Fausto A.M., et al. Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann. Ist Super Sanita. 2019;55:143–150. doi: 10.4415/ANN_19_02_06. [DOI] [PubMed] [Google Scholar]
- 607.Bertolotti L., Tomassone L., Tramuta C., Grego E., Amore G., Ambrogi C., Nebbia P., Mannelli A. Borrelia lusitaniae and spotted fever group rickettsiae in Ixodes ricinus (Acari: Ixodidae) in Tuscany, central Italy. J. Med. Entomol. 2006;43:159–165. doi: 10.1603/0022-2585(2006)043[0159:blasfg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 608.Cinco M., Padovan D., Murgia R., Heldtander M., Engvall E.O. Detection of HGE agent-like Ehrlichia in Ixodes ricinus ticks in northern Italy by PCR. Wien Klin Wochenschr. 1998;110:898–900. [PubMed] [Google Scholar]
- 609.Sanogo Y.O., Parola P., Shpynov S., Camicas J.L., Brouqui P., Caruso G., Raoult D. Genetic Diversity of Bacterial Agents Detected in Ticks Removed from Asymptomatic Patients in Northeastern Italy. Ann. N. Y. Acad. Sci. 2003;990:182–190. doi: 10.1111/j.1749-6632.2003.tb07360.x. [DOI] [PubMed] [Google Scholar]
- 610.Bertola M., Montarsi F., Obber F., Da Rold G., Carlin S., Toniolo F., Porcellato E., Falcaro C., Mondardini V., Ormelli S., et al. Occurrence and Identification of Ixodes ricinus Borne Pathogens in Northeastern Italy. Pathogens. 2021;10:1181. doi: 10.3390/pathogens10091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Cinco M., Banfi E., Trevisan G., Stanek G. Characterization of the first tick isolate of Borrelia burgdorferi from Italy. APMIS. 1989;97:381–382. doi: 10.1111/j.1699-0463.1989.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 612.Castro L.R., Gabrielli S., Iori A., Cancrini G. Molecular detection of Rickettsia, Borrelia, and Babesia species in Ixodes ricinus sampled in northeastern, central, and insular areas of Italy. Exp. Appl. Acarol. 2015;66:443–452. doi: 10.1007/s10493-015-9899-y. [DOI] [PubMed] [Google Scholar]
- 613.Mannelli A., Nebbia P., Tramuta C., Grego E., Tomassone L., Ainardi R., Venturini L., de Meneghi D., Meneguz P.G. Borrelia burgdorferi sensu lato infection in larval Ixodes ricinus (Acari: Ixodidae) feeding on blackbirds in northwestern Italy. J. Med. Ѐntomol. 2005;42:168–175. doi: 10.1093/jmedent/42.2.168. [DOI] [PubMed] [Google Scholar]
- 614.Cinco M., Padovan D., Murgia R., Frusteri L., Maroli M., van de Pol I., Verbeek-De Kruif N., Rijpkema S., Taggi F. Prevalence of Borrelia burgdorferi infection in Ixodes ricinus in central Italy. Eur. J. Clin. Microbiol. Infect. Dis. 1998;17:134–135. doi: 10.1007/bf01682174. [DOI] [PubMed] [Google Scholar]
- 615.Mori E., Pisanu B., Zozzoli R., Solano E., Olivieri E., Sassera D., Montagna M. Arthropods and associated pathogens from native and introduced rodents in Northeastern Italy. Parasitol. Res. 2018;117:3237–3243. doi: 10.1007/s00436-018-6022-4. [DOI] [PubMed] [Google Scholar]
- 616.Sgroi G., Iatta R., Lia R.P., Napoli E., Buono F., Bezerra-Santos M.A., Veneziano V., Otranto D. Tick exposure and risk of tick-borne pathogens infection in hunters and hunting dogs: A citizen science approach. Transbound. Emerg. Dis. 2022;69:E386–E393. doi: 10.1111/tbed.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Stefanelli S., Paladini A., Conforti P.L., Leoncini F., Vigano S., De Giovannini R., Cinco M. Isolation of Borrelia burgdorferi in Tuscany (Italy) New Microbiol. 1994;17:333–336. [PubMed] [Google Scholar]
- 618.Diaz-Sanchez S., Hernandez-Jarguin A., Torina A., de Mera I.G.F., Blanda V., Caracappa S., Gortazar C., de la Fuente J. Characterization of the bacterial microbiota in wild-caught Ixodes ventalloi. Ticks Tick Borne Dis. 2019;10:336–343. doi: 10.1016/j.ttbdis.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 619.Amore G., Tomassone L., Grego E., Ragagli C., Bertolotti L., Nebbia P., Rosati S., Mannelli A. Borrelia lusitaniae in immature Ixodes ricinus (Acari: Ixodidae) feeding on common wall lizards in Tuscany, central Italy. J. Med. Entomol. 2007;44:303–307. doi: 10.1603/0022-2585(2007)44[303:bliiir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 620.Cafiso A., Olivieri E., Floriano A.M., Chiappa G., Serra V., Sassera D., Bazzocchi C. Investigation of Tick-Borne Pathogens in Ixodes ricinus in a Peri-Urban Park in Lombardy (Italy) Reveals the Presence of Emerging Pathogens. Pathogens. 2021;10:732. doi: 10.3390/pathogens10060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Pistone D., Pajoro M., Fabbi M., Vicari N., Marone P., Genchi C., Novati S., Sassera D., Epis S., Bandi C. Lyme borreliosis, Po River Valley, Italy. Emerg. Infect. Dis. 2010;16:1289–1291. doi: 10.3201/eid1608.100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Mancini F., Di Luca M., Toma L., Vescio F., Bianchi R., Khoury C., Marini L., Rezza G., Ciervo A. Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann. Agric. Environ. Med. 2014;21:723–727. doi: 10.5604/12321966.1129922. [DOI] [PubMed] [Google Scholar]
- 623.Grego E., Bertolotti L., Peletto S., Amore G., Tomassone L., Mannelli A. Borrelia lusitaniae OspA Gene Heterogeneity in Mediterranean Basin Area. J. Mol. Evol. 2007;65:512–518. doi: 10.1007/s00239-007-9029-5. [DOI] [PubMed] [Google Scholar]
- 624.Cerutti F., Modesto P., Rizzo F., Cravero A., Jurman I., Costa S., Giammarino M., Mandola M.L., Goria M., Radovic S., et al. The microbiota of hematophagous ectoparasites collected from migratory birds. PLoS ONE. 2018;13:e0202270. doi: 10.1371/journal.pone.0202270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Mantelli B., Pecchioli E., Hauffe H.C., Rosa R., Rizzoli A. Prevalence of Borrelia burgdorferi s.l. and Anaplasma phagocytophilum in the wood tick Ixodes ricinus in the Province of Trento, Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25:737–739. doi: 10.1007/s10096-006-0208-x. [DOI] [PubMed] [Google Scholar]
- 626.Tomassone L., Ceballos L.A., Ragagli C., Martello E., De Sousa R., Stella M.C., Mannelli A. Importance of Common Wall Lizards in the Transmission Dynamics of Tick-Borne Pathogens in the Northern Apennine Mountains, Italy. Microb. Ecol. 2017;74:961–968. doi: 10.1007/s00248-017-0994-y. [DOI] [PubMed] [Google Scholar]
- 627.Rosa R., Andreo V., Tagliapietra V., Barákova I., Arnoldi D., Hauffe H.C., Manica M., Rosso F., Blanarova L., Bona M., et al. Effect of Climate and Land Use on the Spatio-Temporal Variability of Tick-Borne Bacteria in Europe. Int. J. Environ. Res. Public Health. 2018;15:732. doi: 10.3390/ijerph15040732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Capelli G., Ravagnan S., Montarsi F., Ciocchetta S., Cazzin S., Porcellato E., Babiker A.M., Cassini R., Salviato A., Cattoli G., et al. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: A cost-effectiveness analysis in north-eastern Italy. Parasites Vectors. 2012;5:61. doi: 10.1186/1756-3305-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Piccolin G., Benedetti G., Doglioni C., Lorenzato C., Mancuso S., Papa N., Pitton L., Ramon M.C., Zasio C., Bertiato G. A study of the presence of B. burgdorferi, Anaplasma (previously Ehrlichia) phagocytophilum, Rickettsia, and Babesia in Ixodes ricinus collected within the territory of Belluno, Italy. Vector Borne Zoonotic Dis. 2006;6:24–31. doi: 10.1089/vbz.2006.6.24. [DOI] [PubMed] [Google Scholar]
- 630.Carpi G., Cagnacci F., Wittekindt N.E., Zhao F., Qi J., Tomsho L.P., Drautz D.I., Rizzoli A., Schuster S.C. Metagenomic Profile of the Bacterial Communities Associated with Ixodes ricinus Ticks. PLoS ONE. 2011;6:e25604. doi: 10.1371/journal.pone.0025604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Mannelli A., Boggiatto G., Grego E., Cinco M., Murgia R., Stefanelli S., DE Meneghi D., Rosati S. Acarological risk of exposure to agents of tick-borne zoonoses in the first recognized Italian focus of Lyme borreliosis. Epidemiol. Infect. 2003;131:1139–1147. doi: 10.1017/s0950268803001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Rizzoli A., Merler S., Furlanello C., Genchi C. Geographical information systems and bootstrap aggregation (bagging) of tree-based classifiers for Lyme disease risk prediction in Trentino, Italian Alps. J. Med. Ѐntomol. 2002;39:485–492. doi: 10.1603/0022-2585-39.3.485. [DOI] [PubMed] [Google Scholar]
- 633.Ruzic-Sablijic E., Strle F., Cimperman J. The Ixodes ricinus tick as a vector of Borrelia burgdorferi in Slovenia. Eur. J. Epidemiol. 1993;9:396–400. doi: 10.1007/bf00157396. [DOI] [PubMed] [Google Scholar]
- 634.Strle F., Cheng Y., Nelson J.A., Picken M.M., Bouseman J.K., Picken R.N. Infection rate of Ixodes ricinus ticks with Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto in Slovenia. Eur. J. Clin. Microbiol. Infect. Dis. 1995;14:994–1001. doi: 10.1007/bf01691382. [DOI] [PubMed] [Google Scholar]
- 635.Karasartova D., Gureser A.S., Gokce T., Celebi B., Yapar D., Keskin A., Celik S., Ece Y., Erenler A.K., Usluca S., et al. Bacterial and protozoal pathogens found in ticks collected from humans in Corum province of Turkey. PLoS Neglected Trop. Dis. 2018;12:e0006395. doi: 10.1371/journal.pntd.0006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Sen E., Uchishima Y., Okamoto Y., Fukui T., Kadosaka T., Ohashi N., Masuzawa T. Molecular detection of Anaplasma phagocytophilum and Borrelia burgdorferi in Ixodes ricinus ticks from Istanbul metropolitan area and rural Trakya (Thrace) region of north-western Turkey. Ticks Tick Borne Dis. 2011;2:94–98. doi: 10.1016/j.ttbdis.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 637.Ji Z., Jian M., Yue P., Cao W., Xu X., Zhang Y., Pan Y., Yang J., Chen J., Liu M., et al. Prevalence of Borrelia burgdorferi in Ixodidae Tick around Asia: A Systematic Review and Meta-Analysis. Pathogens. 2022;11:143. doi: 10.3390/pathogens11020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Orkun O., Cakmak A., Nalbantoglu S., Karaer Z. Turkey tick news: A molecular investigation into the presence of tick-borne pathogens in host-seeking ticks in Anatolia; Initial evidence of putative vectors and pathogens, and footsteps of a secretly rising vector tick, Haemaphysalis parva. Ticks Tick Borne Dis. 2020;11:101373. doi: 10.1016/j.ttbdis.2020.101373. [DOI] [PubMed] [Google Scholar]
- 639.Orkun O. Comprehensive screening of tick-borne microorganisms indicates that a great variety of pathogens are circulating between hard ticks (Ixodoidea: Ixodidae) and domestic ruminants in natural foci of Anatolia. Ticks Tick Borne Dis. 2022;13:102027. doi: 10.1016/j.ttbdis.2022.102027. [DOI] [PubMed] [Google Scholar]
- 640.Zhioua E., Bouattour A., Hu C.M., Gharbi M., Aeschliman A., Ginsberg H.S., Gern L. Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi sensu lato in North Africa. J. Med. Ѐntomol. 1999;36:216–218. doi: 10.1093/jmedent/36.2.216. [DOI] [PubMed] [Google Scholar]
- 641.Boucheikhchoukh M., Laroche M., Aouadi A., Dib L., Benakhla A., Raoult D., Parola P. MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018;57:39–49. doi: 10.1016/j.cimid.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 642.Toledo A., Anda P., Escudero R., Larsson C., Bergstrom S., Benach J.L. Phylogenetic Analysis of a Virulent Borrelia Species Isolated from Patients with Relapsing Fever. J. Clin. Microbiol. 2010;48:2484–2489. doi: 10.1128/jcm.00541-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Boyer P.H., Koetsveld J., Zilliox L., Sprong H., Talagrand-Reboul E., Hansmann Y., de Martino S.J., Boulanger N., Hovius J.W., Jaulhac B. Assessment of Borrelia miyamotoi in febrile patients and ticks in Alsace, an endemic area for Lyme borreliosis in France. Parasites Vectors. 2020;13:199. doi: 10.1186/s13071-020-04071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Franck M., Ghozzi R., Pajaud J., Lawson-Hogban N.E., Mas M., Lacout A., Perronne C. Borrelia miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020;7:55. doi: 10.3389/fmed.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Nicholson F.D. Tick Fever in Palestine. Br. Med. J. 1919;2:811. doi: 10.1136/bmj.2.3077.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.McNamara J.J., Kay H.H. Relapsing fever (Borrelia) in an adolescent tourist in Israel. J. Adolesc. Health Care. 1988;9:421–423. doi: 10.1016/0197-0070(88)90042-3. [DOI] [PubMed] [Google Scholar]
- 647.Wengrower D., Knobler H., Gillis S., Chajek-Shaul T. Myocarditis in tick-borne relapsing fever. J. Infect. Dis. 1984;149:1033. doi: 10.1093/infdis/149.6.1033. [DOI] [PubMed] [Google Scholar]
- 648.Sidi G., Davidovitch N., Balicer R.D., Anis E., Grotto I., Schwartz E. Tickborne relapsing fever in Israel. Emerg. Infect. Dis. 2005;11:1784–1786. doi: 10.3201/eid1111.050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Shaked Y., Maier M.K., Samra Y. Relapsing fever and salmonella bacteraemia simultaneously affecting a healthy young man. J. Infect. 1986;13:308–309. doi: 10.1016/s0163-4453(86)91718-4. [DOI] [PubMed] [Google Scholar]
- 650.Eisenberg S., Gunders A.E., Cohen A.M. Tick-borne relapsing fever in the Judean Hills, including a case with massive haematuria. Trans. R. Soc. Trop. Med. Hyg. 1968;62:679–681. doi: 10.1016/0035-9203(68)90119-3. [DOI] [PubMed] [Google Scholar]
- 651.Kahouli S., Naoui H., Uwingabiye J., Reggad A., Ennibi K., Bouchrik M., Lmimouni B.E. Relapsing fever in a Moroccan man. Med. Sante Trop. 2018;28:141–143. doi: 10.1684/mst.2018.0792. [DOI] [PubMed] [Google Scholar]
- 652.Salant H., Nachum-Biala Y., Zivotofsky D., Tzur T.E., Baneth G. Babesia negevi infection in dogs and response to treatment. Ticks Tick Borne Dis. 2024;15:102282. doi: 10.1016/j.ttbdis.2023.102282. [DOI] [PubMed] [Google Scholar]
- 653.Schwarzer S., Margos G., Overzier E., Fingerle V., Baneth G., Straubinger R.K. Borrelia persica: In vitro cultivation and characterization via conventional PCR and multilocus sequence analysis of two strains isolated from a cat and ticks from Israel. Ticks Tick Borne Dis. 2015;6:751–757. doi: 10.1016/j.ttbdis.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 654.Boularias G., Azzag N., Galon C., Simo L., Boulouis H.-J., Moutailler S. High-Throughput Microfluidic Real-Time PCR for the Detection of Multiple Microorganisms in Ixodid Cattle Ticks in Northeast Algeria. Pathogens. 2021;10:362. doi: 10.3390/pathogens10030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Elbir H., FotsoFotso A., Diatta G., Trape J.F., Arnathau C., Renaud F., Durand P. Ubiquitous bacteria Borrelia crocidurae in Western African ticks Ornithodoros sonrai. Parasites Vectors. 2015;8:477. doi: 10.1186/s13071-015-1089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.