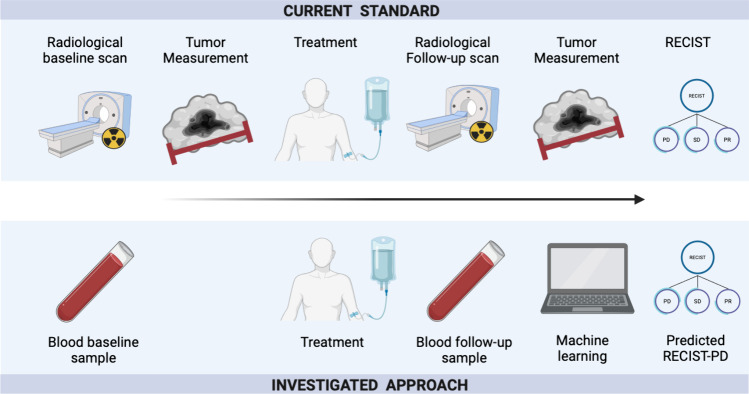
Abstract
Purpose
In this study, we aimed to evaluate the potential of routine blood markers, serum tumour markers and their combination in predicting RECIST-defined progression in patients with stage IV non-small cell lung cancer (NSCLC) undergoing treatment with immune checkpoint inhibitors.
Methods
We employed time-varying statistical models and machine learning classifiers in a Monte Carlo cross-validation approach to investigate the association between RECIST-defined progression and blood markers, serum tumour markers and their combination, in a retrospective cohort of 164 patients with NSCLC.
Results
The performance of the routine blood markers in the prediction of progression free survival was moderate. Serum tumour markers and their combination with routine blood markers generally improved performance compared to routine blood markers alone. Elevated levels of C-reactive protein (CRP) and alkaline phosphatase (ALP) ranked as the top predictive routine blood markers, and CYFRA 21.1 was consistently among the most predictive serum tumour markers. Using these classifiers to predict overall survival yielded moderate to high performance, even when cases of death-defined progression were excluded. Performance varied across the treatment journey.
Conclusion
Routine blood tests, especially when combined with serum tumour markers, show moderate predictive value of RECIST-defined progression in NSCLC patients receiving immune checkpoint inhibitors. The relationship between overall survival and RECIST-defined progression may be influenced by confounding factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-024-05814-2.
Keywords: Blood-based markers, Progression-free survival, RECIST, NSCLC, Machine learning, Immunotherapy
Introduction
Clinical trials are necessary to evaluate the efficacy and safety of new drugs in the treatment of non-small cell lung cancer (NSCLC). Overall survival (OS) is a desirable endpoint for assessing the effectiveness of cancer therapy (Driscoll and Rixe 2009). However, OS requires a lengthy follow-up, and death can occur due to various causes unrelated to cancer treatment. As an alternative, endpoints based on tumour size changes, such as progression-free survival (PFS), can be measured at earlier time points and have often replaced OS as an endpoint in clinical trials (Mushti et al. 2018). An accurate definition and assessment of disease progression are, therefore, vital in evaluating treatment efficacy in NSCLC patients. The Response Evaluation Criteria in Solid Tumours (RECIST) (Eisenhauer et al. 2009) is a commonly used framework to define progression, defined as either an increase in the size of target lesions (above 20%), an unequivocal increase of non-target lesions’ size, appearance of new lesions, or death. In immunotherapy, progression must be reassessed and confirmed in the subsequent follow-up (Seymour et al. 2017).
Routine blood tests are not invasive and are easily accessible as part of the routine monitoring of cancer patients during treatment (Bilen et al. 2019; Farina et al. 2023). Given that these are an already established tool in standard cancer patient management, leveraging them for the prediction of progression according to RECIST could offer a potentially cheaper and faster method to identify progression, compared to imaging assessments.
In NSCLC, research on the association between routine blood markers and progression has focused on inflammatory markers (Tanizaki et al. 2018; Iivanainen et al. 2019; Inomata et al. 2020; Peng et al. 2020; Chen et al. 2023), including the C reactive protein (CRP) and the neutrophil/lymphocyte ratio, lactate dehydrogenase (LDH) (Tanizaki et al. 2018; Inomata et al. 2020; Zhang et al. 2022) and albumin (Li et al. 2022; Menekse et al. 2023). Zhou et al. (2022) found good performance in the prediction of fast progression (significant progression occurring soon after the initiation of treatment) by a support vector machine model trained on laboratory test values, specifically on a four biomarker panel composed of neutrophil, CRP, LDH and alanine aminotransaminase (ALT). Fast progression patients predicted by this model were also found to have poor OS and PFS.
Serum tumour markers, produced by or in response to the tumour, have also been studied for monitoring both response and non-response, as well as for assessing the outcome of early treatment in immunotherapy-treated advanced-stage NSCLC (Essink et al. 2016; Lang et al. 2019; Dall’Olio et al. 2020; Muller et al. 2021; van Delft et al. 2022).
The objective of this study is to evaluate the potential of routine blood values in the prediction of RECIST progression in a longitudinal cohort of NSCLC patients receiving immune checkpoint inhibitors and to determine if combining routine with serum tumour markers offers additional advantages.
Materials and methods
Study cohort
We retrospectively collected stage-IV NSCLC patients treated with checkpoint inhibitors at the Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital (NKI-AVL; Amsterdam, The Netherlands) between 2014 and 2016, as a multi-modal (i.e. radiological + blood values) expansion of the dataset presented by Trebeschi et al. (2021). Patient and treatment characteristics are reported in Table 1. We retrieved blood data acquired for these patients between 2013 and 2018, collecting all routinely available measurements of blood markers from routine blood tests and serum tumour markers measured in the blood. A complete list of the markers collected can be accessed in the supplements. Progression-free survival was calculated from start of treatment to the date of RECIST 1.1 progression or death.
Table 1.
Cohort characteristics
| Age | |
| Median (years) (min, max) | 64 (37, 83) |
| Sex | |
| Male | 96 (58.5%) |
| Female | 68 (41.5%) |
| Tumor location diagnosis | |
| Upper lobe, bronchus or lung | 67 |
| Lower lobe, bronchus or lung | 33 |
| Bronchus or lung, unspecified | 10 |
| Middle lobe, bronchus or lung | 4 |
| Main bronchus | 4 |
| Multiple locations | 10 |
| Unknown | 36 |
| Treatment | |
| Nivolumab | 164 |
| Outcomes | |
| Progression | 139 (85%) |
| Death | 128 (78%) |
| Median time to progression (days) | 118 |
| Median time to death (days) | 303 |
Time-varying regression analysis
In order to investigate whether blood markers were linked to RECIST progression during treatment, we used a statistical model named time-varying Cox regression (t-vCR) (Zhang et al. 2018). This model considers the entire time period between the start of treatment and the date when progression occurred, and analyses fluctuations in blood values during that time period to determine if they are significantly related to progression-free survival. The hazard ratio of each covariate (each blood value) will reflect the overall effect on the hazard rate considering that the covariate’s value may vary over time. Non-numerical blood values were filtered out or replaced by lower or upper bound values whenever applicable, as detailed in the supplements. Blood tests containing at least 25% of the available markers were used. We addressed remaining missing data using an unsupervised, iterative imputer based on Bayesian Ridge regression (Buck 1960; van Buuren and Groothuis-Oudshoorn 2011). To ensure convergence of the t-vCR model, we normalised the variables to a 0–1 range using the MinMaxScaler from Scikit-learn (Pedregosa et al. 2011).
Machine learning pipeline
To study if these markers could predict progression, we employed several classifiers, including random forests (RF) (Breiman 2001), and we trained them to predict whether progression occurs concurrently or will occur within different future time points: the next month, the next three, six, nine and twelve months from the date of the individual blood test. Each endpoint was trained and validated independently. Hyperparameter tuning was conducted for a subset of random forest parameters in all cross-validation splits, as detailed in the supplements. To predict concurrent progression (at the time of blood test acquisition), we incorporated all blood tests conducted from the start of treatment until three months after the recorded RECIST progression. For predicting progression at future time points, we included all blood tests conducted between three months prior to the start of treatment and up to three months after (or until the recorded progression, whichever happened first). Missing data was addressed using the same approach as detailed in the preceding section. Shapely Additive exPlanation (SHAP) values were used to explain the decisions of the RF classifier based on the validation sets (Lundberg et al. 2020). We used Python 3.9.7 for our analysis. Scikit-learn 1.0.2 (Pedregosa et al. 2011), Lifelines 0.27.4 (Creators Davidson-Pilon 2022), NumPy 1.21.6, Pandas 1.3.5 and SHAP 0.41.0 (Lundberg et al. 2020) were used for the implementation of the methods. To assess the robustness of the findings, we also repeated the analysis replacing the RF with support vector machines (SVM) (Hearst et al. 1998), and logistic regression. The study design is shown in Fig. 1.
Fig. 1.
Scheme of the study design
Validation strategy
Patient timelines can differ significantly, and traditional methods such as train/test splits or K-fold cross-validation on smaller sets may produce biased results depending on the specific split used. To avoid such biases, we utilised Monte Carlo cross-validation (MCCV) to estimate model performance (Xu and Liang 2001). With MCCV, we can randomly and reproducibly divide the dataset into multiple training (80%) and validation (20%) sets a certain number of times until stable and consistent performance estimates are obtained. This approach helps to ensure a reliable and unbiased evaluation of the model's performance. In our case, splitting is done on a patient-level on the raw data, after imputation of missing values. Results are reported in median performance (area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), at the median classification probability) across 100 MCCV folds with 95% confidence intervals. The code is shared publicly.1
Results
Routine blood markers were available for n = 164 patients. The median age of the patients was 64 years, and 58.5% were male. Radiological progression was recorded in n = 139 patients. Serum tumour markers were available for n = 70 patients (43%), showing similar demographics: a median age of 64 years and 63% being male. The dataset for routine blood markers consisted of 3923 longitudinal routine blood tests, with a median of 21 blood tests (each blood test containing the measurements of multiple markers) collected from each patient at a median interval of approximately 14 days. A complete list of the utilised 31 routine blood markers and their abbreviations are reported in Supplementary Table 1. The subset of tumour markers dataset consisted of 1509 longitudinal blood tests, with each patient receiving a median of 20 tests at approximately 25-day median intervals. A total of five serum tumour markers were included: cytokeratin 19 fragment antigen (CYFRA 21.1), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cancer antigen 125 (CA125), and squamous cell carcinoma antigen (SCC), with the first three being the most prevalent in our dataset. Validation details for each endpoint in each MCCV fold are reported in the supplements.
Association between blood markers and PFS
Time-varying regression analysis was employed to study longitudinal links between blood markers and progression (Supplementary Tables 2 and 3). In routine blood values, increases in the levels of C-reactive protein (CRP) and alkaline phosphatase (ALP) were significantly associated with progression events (HR = 3.68, and 7.18; p = 0.018, and 0.018 respectively; Table 2). When combined with tumour markers, these blood values kept the same positive trends, but only CRP maintained its statistical significance (p = 0.011). Furthermore, CYFRA 21.1 also showed statistical significance (HR = 22.26, p = 0.008). The hazard ratios of the variables significantly associated with PFS are described in Table 2.
Table 2.
Results of the time-varying Cox regression analysis
| PFS | ||
|---|---|---|
| Marker | HR (95% CI) | p-value |
| Routine blood markers | ||
| CRP | 3.68 (1.25–10.83) | 0.018 |
| ALP | 7.18 (1.39–37.03) | 0.018 |
| Routine and tumour markers | ||
| CRP | 6.64 (1.54–28.63) | 0.011 |
| CYFRA 21.1 | 22.26 (2.28–217.41) | 0.008 |
Only variables with significant effects for PFS in routine blood markers or in combination with tumour markers are shown and compared. HR = hazard ratio; CI = confidence interval. The effects of the remaining variables are provided in the supplementary tables 3 and 4
To quantify the predictive value of blood markers to identify concurrent radiological progression, we used machine learning approaches. From now on, we refer to the results of the RF classifier. The results of SVMs and logistic regressions can be found in Supplementary Table 4, with comparable trends. Using only routine blood markers, we observed a moderate predictive value of 0.67 AUC (CI: 0.60–0.74). A similar result was observed when employing tumour markers, with an AUC of 0.67 (CI: 0.54–0.79). Combining routine blood and tumour markers increased the AUC to 0.69 (CI: 0.55–0.82). The AUC-based performance of the RF machine learning models is detailed in Table 3. Supporting metrics are provided in Supplementary Table 6. Using SHAP to uncover the most important markers for the machine learning models based on RFs (see Fig. 2a and Table 4), we observed ALP and CRP to be among the top most predictive markers for both models based on routine blood markers alone and on combined routine blood and tumour markers, and CYFRA 21.1 to follow in importance in the combined routine blood and tumour markers, suggesting an association of these markers with progression. SHAP summary plots for all prediction endpoints are included in the supplements (Supplementary Figs. 1–12).
Table 3.
PFS and OS prediction performance using routine blood markers with/out combining tumour markers, with/out including death-defined progression cases
| Data | Time point | AUC(95% CI) | |||
|---|---|---|---|---|---|
| PFS | OS | PFS (excluding death as PD) | OS (excluding death as PD) | ||
| Routine markers | Concurrent | 0.67 (0.60–0.74) | – | – | – |
| 1-month | 0.74 (0.65–0.82) | 0.85 (0.75–0.93) | 0.71 (0.61–0.81) | 0.84 (0.73–0.93) | |
| 3-month | 0.75 (0.69–0.81) | 0.79 (0.72–0.85) | 0.73 (0.66–0.8) | 0.79 (0.68–0.87) | |
| 6-month | 0.70 (0.64–0.77) | 0.70 (0.64–0.77) | 0.71 (0.64–0.78) | 0.71 (0.63–0.78) | |
| 9-month | 0.71 (0.65–0.78) | 0.70 (0.63–0.76) | 0.76 (0.68–0.82) | 0.74 (0.67–0.81) | |
| 12-month | 0.69 (0.62–0.76) | 0.71 (0.65–0.77) | 0.74 (0.66–0.81) | 0.76 (0.69–0.82) | |
| Tumour markers | Concurrent | 0.67 (0.54–0.79) | – | – | – |
| 1-month | 0.74 (0.54–0.9) | 0.69 (0.52–0.85) | 0.72 (0.53–0.88) | 0.66 (0.54–0.78) | |
| 3-month | 0.79 (0.67–0.9) | 0.82 (0.68–0.93) | 0.77 (0.63–0.89) | 0.82 (0.67–0.94) | |
| 6-month | 0.77 (0.66–0.87) | 0.77 (0.66–0.87) | 0.76 (0.64–0.86) | 0.74 (0.61–0.85) | |
| 9-month | 0.78 (0.68–0.87) | 0.74 (0.63–0.85) | 0.77 (0.64–0.87) | 0.73 (0.6–0.84) | |
| 12-month | 0.71 (0.59–0.82) | 0.74 (0.62–0.84) | 0.75 (0.63–0.86) | 0.75 (0.62–0.86) | |
| Routine + tumour markers | Concurrent | 0.69 (0.55–0.82) | – | – | – |
| 1-month | 0.83 (0.67–0.95) | 0.87 (0.75–0.96) | 0.83 (0.67–0.95) | 0.95 (0.9–0.98) | |
| 3-month | 0.86 (0.74–0.95) | 0.89 (0.77–0.97) | 0.81 (0.66–0.93) | 0.88 (0.76–0.96) | |
| 6-month | 0.83 (0.71–0.92) | 0.78 (0.65–0.89) | 0.80 (0.66–0.91) | 0.74 (0.58–0.88) | |
| 9-month | 0.79 (0.66–0.89) | 0.76 (0.63–0.87) | 0.78 (0.63–0.90) | 0.74 (0.59–0.87) | |
| 12-month | 0.72 (0.57–0.85) | 0.76 (0.63–0.87) | 0.73 (0.57–0.87) | 0.77 (0.62–0.89) | |
Fig. 2.
a Venn diagrams showing blood markers found related to RECIST progression across different analyses, namely the top predictive values for the SHAP analysis and the significant values for the time varying model analysis b Frequency of missing values in the dataset
Table 4.
Most predictive blood markers (in order of importance) for RECIST-based PFS prediction according to SHAP using routine blood data alone and combined with serum tumour markers
| Data | Time point | Predictive marker 1 | Predictive marker 2 | Predictive marker 3 | Predictive marker 4 | Predictive marker 5 |
|---|---|---|---|---|---|---|
| Predicting progression-free survival | ||||||
| Routine blood markers | Current | AST | ALP | GFR | CRP | Plt |
| 1-month | ALP | Albumin | ImmGran | MCV | AST | |
| 3-month | ALP | CRP | AST | Chloride | WBC | |
| 6-month | ALP | Plt | Chloride | CRP | AST | |
| 9-month | ALP | CRP | Plt | Cr | AST | |
| 12-month | ALP | CRP | AST | Chloride | RBC | |
| Routine and tumour markers | Current | ALP | CYFRA 21.1 | Chloride | CRP | SCC |
| 1-month | ALP | CYFRA 21.1 | SCC | CA125 | NSE | |
| 3-month | CYFRA 21.1 | CA125 | Plt | ALP | Ht | |
| 6-month | CYFRA 21.1 | CA125 | Plt | CRP | Ht | |
| 9-month | CA125 | CYFRA 21.1 | SCC | CRP | AST | |
| 12-month | ALP | CYFRA 21.1 | Chloride | CRP | SCC | |
| Excluding death as progression | ||||||
| Routine blood markers | 1-month | ALP | Albumin | CRP | MCV | AST |
| 3-month | ALP | CRP | Chloride | AST | Plt | |
| 6-month | ALP | Chloride | Plt | AST | CRP | |
| 9-month | ALP | Cr | GFR | CRP | Plt | |
| 12-month | ALP | RBC | CRP | AST | Chloride | |
| Routine and tumour markers | 1-month | ALP | CRP | Magnesium | WBC | CYFRA 21.1 |
| 3-month | ALP | CYFRA 21.1 | SCC | CA125 | CRP | |
| 6-month | CYFRA 21.1 | CA125 | Plt | ALP | Ht | |
| 9-month | CYFRA 21.1 | CA125 | Plt | Ht | Magnesium | |
| 12-month | CA125 | CYFRA 21.1 | SCC | ALP | AST | |
Relation between predictive blood markers and PFS
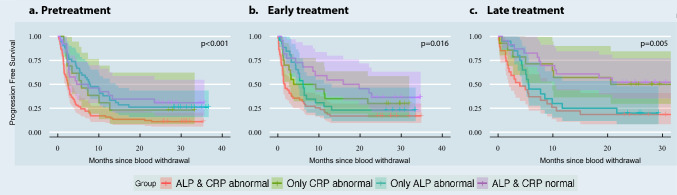
Given the frequency of ALP and CRP emerging as important markers across the results of different types of RF analysis, we investigated further their relationship with PFS. We stratified the population of lab exams into subgroups based on the cutoff values used in our clinical workflow to define normal/abnormal ALP and CRP values: i.e. ALP > = 98 U/l for females, 115 U/l for males, and CRP > = 8 mg/l. This split created four subgroups: (1) normal-ALP normal-CRP, (2) normal-CRP abnormal-ALP, (3) abnormal-CRP normal-ALP, and (4) abnormal-ALP normal-CRP. Moreover, we split the analysis into the different stages of the treatment timeline, looking separately at the relationship between PFS and blood values collected pre-treatment (maximum one month), early on during the treatment (between 4 and 6 weeks), and at a later stage during treatment (between 10 and 12 weeks). Figure 3 shows the survival curves.
Fig. 3.
Survival KM curves of normal/abnormal CRP and ALP groups, as measured at a before start of treatment, b early treatment and c late during the treatment
The group with abnormal CRP and ALP values showed shorter progression time, compared to the other subgroups, ranging from a median of 52 days for blood samples collected early during treatment to 123 days for blood samples collected later on during treatment (Supplementary Table 7). Interestingly, the normal CRP and ALP group showed lower median PFS pre-treatment (143 days; Fig. 3) than the groups with at most one abnormal value. During treatment, however, normalcy in both values was characteristic of longer PFS, reaching a median of 493 days, compared to 286 days of abnormal ALP group, 190 days of abnormal CRP group, and 52 days of abnormal CRP and ALP. Overall, no clear pattern of behaviour is visible among these groups, suggesting a complex relationship between CRP, ALP, and progression during treatment.
Predicting future progression
To investigate whether blood and tumour markers can predict future progression, we set up the machine learning pipeline to predict whether progression will happen within a given time frame from the moment of the blood sampling. The timeframes investigated were one, three, six, nine and twelve months. To predict progression within one month from the moment of blood withdrawal, the model reached an AUC of 0.74 for both routine markers, as well as tumour markers. The combination of routine markers and tumour markers yielded a higher performance, with an AUC of 0.83. To predict progression within three months from the moment of blood withdrawal, the model reached the highest performance in all three combinations with 0.86 AUC, observed in the combination of routine and tumour markers, being the highest. The performance of the model is then observed to decrease from three months on: from 0.75 to 0.69 AUC to predict progression within the next 12 months in blood markers-only models, from 0.79 to 0.71 AUC in tumour markers-only models, and from 0.86 to 0.72 AUC for combined tumour and blood markers models. Results are displayed in Fig. 4 and Table 3. ALP and CRP were also among the most important markers for predicting future progression, as seen in Table 4. Additional evaluation metrics are provided in Supplementary Table 5. The numbers of progressive patients in all validation sets for all studied endpoints and data modalities is reported in the supplements.
Fig. 4.
Comparative analysis of the performance with respect to a its inputs, and b its outputs
Relationship between PFS and OS
We also investigated the relationship between PFS and OS in our cohort. In order to do that, we quantified the performance of our models, trained to predict PFS (PFS models), in the prediction of OS: if PFS and OS were to be independent variables, the models would not show any predictive value for the OS endpoint. Surprisingly, the models were observed to perform, on average, 3–4% AUC higher in the prediction of OS within three months from the moment of blood withdrawal in all modalities, up to 11% better performance for routine blood markers only models within one month from blood withdrawal (Table 3). Similar performances between PFS and OS were observed for the rest of the timeline.
These findings suggest a significant overlap of progression and the occurrence of death. Because progression in PFS is defined as either RECIST progression or death of the patient, these were analysed separately. We analysed the performance of the PFS models to predict RECIST progression only, and removed from the analysis the patients whose progression was defined by death. The models were retrained to ensure that no influence of death events can be traced into the models. We observed an average drop of 2–3% AUC in predictive performance of the models, up to 5% AUC drop for combined tumour and blood markers models within three months from the blood withdrawal. Similar performances between PFS and OS were observed for the rest of the timeline. Figure 4b shows the change in performances over time.
Discussion
In this study, we aimed to assess the feasibility of using routine blood tests and serum tumour markers as a tool for defining radiological progression-free survival. Routine blood tests are non-invasive, non-ionizing, quantitative measurements not subject to variable interpretation. They are widely and frequently available and easily accessible in hospital settings, making them a compelling option for rapid monitoring of early treatment effects and tracking disease progression. Although genetic analysis of blood samples, such as circulating tumour DNA (ctDNA), has demonstrated the potential to identify radiological progression prior to imaging (Bettegowda et al. 2014; Hellmann et al. 2020), these require technical standardisation (Brozos-Vázquez et al. 2021), specialised equipment and highly trained personnel (Li et al. 2019).
Across all analyses, our findings revealed that, particularly, C-reactive protein (CRP) and alkaline phosphatase (ALP) were associated with radiological progression. In literature, a high level of pre-treatment CRP has been found to be a predictor of worse PFS and OS in NSCLC patients undergoing immunotherapy (Riedl et al. 2020). Moreover, CRP dynamics during treatment have also been found to be informative: early-treatment flares in the levels of CRP predict response (Klümper et al. 2022) and early decreases are associated with reduced risk of progression (Riedl et al. 2020). Additionally, fast increases over time and overall elevated trajectories of CRP have also been found to be predictors of high risk of progression (Riedl et al. 2020). While research on ALP has demonstrated their potential as biomarkers across different tumour types, its role in NSCLC remains uncertain (Jiang et al. 2023), and literature concerning NSCLC patients receiving immunotherapy is limited. Yang et al. (2023) found that higher levels of pre-treatment ALP are associated with lower overall response rate, and shorter median progression-free survival (mPFS) in patients with NSCLC treated with immune checkpoint inhibitors and that ALP is an independent prognostic indicator of mPFS. Julian et al. (2022) found that high levels of ALP were a risk increasing prognostic factor in advanced NSCLC patients receiving anti-PD-1/PD-L1 immune checkpoint inhibitors. In all analyses conducted in this study, there was a noticeable improvement in the predictive performance when routine blood and tumour markers were combined, reaching its peak at three months, compared to their individual performances. This highlights the complementary nature of the various blood-based biomarkers and the potential added value of incorporating them together. In the study of van Delft et al. (2022), CYFRA 21.1 was identified as the most predictive tumour marker for radiological response prediction at 6 months after treatment. Our study aligns with this finding for the prediction of radiological progression using combined blood markers, where CYFRA 21.1 consistently appears among the top two predictive markers across all time points. Despite the evident value of tumour markers, we nonetheless observed that ALP was more predictive (by SHAP) than CYFRA 21.1 for short-term events but also at one-year events. This example also shows how integrative analyses can be insightful in comparing different biomarkers with each other with respect to treatment endpoints. Integrating routine blood and tumour marker analysis with advanced techniques such as mass cytometry (CyTOF), that offer a comprehensive analysis of cellular phenotypes and functions at the single-cell level could also be relevant. CyTOF has emerged recently for the identification of circulating immune cell features associated with response (Mueller et al. 2022), and survival and progression outcomes (Rochigneux et al. 2022) of NSCLC patients receiving immunotherapy.
In a subsequent analysis, we looked further into the relationship between PFS and overall survival (OS) endpoints. Interestingly, our results indicated that the models tailored for PFS were more predictive for OS outcomes than anticipated in certain prediction time points. Routine blood parameters showed higher predictive power with shorter prediction windows, suggesting they may reflect the patient's ongoing overall health status, and other underlying conditions or acute illnesses not exclusively related to cancer (Crosbie et al. 2013). Conversely, tumour markers had an opposite pattern, with increasing predictive performance for longer prediction windows, implying that they may be more indicative of the early stages of tumour progression and its trajectory.
Indeed, the relationship between PFS and OS is complex and varies across different cancer types and therapeutics. In the context of NSCLC and immunotherapy or targeted therapies, previous studies suggest, at most, moderate potential for PFS to be used as a surrogate of OS (Ye et al. 2020; Shameer et al. 2021; Hua et al. 2022), while strongly advocating for the need for patient-level analyses, where true surrogacy can be evaluated.
Unlike OS, PFS, as assessed by RECIST, is subject to variability due to the inconsistent application of the criteria. The variability sources can pertain to, among others, reader experience (Tovoli et al. 2018), identification of new lesions (Beaumont et al. 2018), unidimensional measurement errors (Yoon et al. 2016), selection of target lesions (Iannessi et al. 2021) or non-target disease assessment (Morse et al. 2019). While some of these can be minimised by, for example, having automated methods for lesion measurements (Fournier et al. 2021) and multiple experienced readers (Karmakar et al. 2019; Schmid et al. 2021), the reader-subjective nature of RECIST, limited to the selection of target lesions, may not be eliminated. Without the guarantee of an objective definition of progression, PFS may be misidentified, potentially introducing noise into our data.
Our study has some limitations. Firstly, this was a single-centre study with no external validation, implying that our findings might not be directly translatable or generalisable to external cohorts without further testing. To mitigate this, we report the average performance of our models across 100 cross-validation splits. Moreover, the specificity of our cohort to NSCLC and its treatment with immunotherapy may limit the applicability of our results to other types of cancers or treatments. Larger cohorts, with appropriate sample size estimations and additional study contexts, will be required to confirm our results.
Conclusions
Our study highlights the potential of using routine blood tests as a tool for predicting progression free survival in NSCLC cancer patients receiving immune checkpoint inhibitors. Integrating routine blood markers and serum tumour markers within machine learning models may enable more performant and timely detection of disease progression, ultimately aiding clinical decision-making. Further studies with larger cohorts are needed to validate these findings and explore the generalizability of our approach to other cancer types and treatment modalities.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
MY study design, machine learning and statistical analysis, implementation, validation, manuscript writing. TTM study design, statistical analysis, implementation, validation, manuscript writing. MdB data collection and validation. AS data collection and validation. ZB study design, validation, manuscript writing. EFS study design. RBT study design. DvdB study design. ST study design, project supervision, validation, manuscript writing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author, S.T., upon reasonable request.
Declarations
Conflict of interest
The author(s) declare no conflict of interest.
Consent to participate
Informed consent was obtained from all subjects and/or their legal guardian(s) to use their data for scientific research.
Consent to publication
Not applicable.
Ethical approval
This study was approved by the International Review Board (IRB) from the Netherlands Cancer Institute - Antoni van Leeuwenhoek Hospital (IRB), and complies with the Declaration of Helsinki.
Footnotes
M Yeghaian and T Tareco Bucho are co-first authors and contributed equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Beaumont H et al (2018) Discrepancies of assessments in a RECIST 1.1 phase II clinical trial—association between adjudication rate and variability in images and tumors selection. Cancer Imaging 18(1):50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettegowda C et al (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6(224):224ra24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen MA et al (2019) The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer 125(1):127–134 [DOI] [PubMed] [Google Scholar]
- Breiman L (2001) Random forests. Mach Learn 45(1):5–32 [Google Scholar]
- Brozos-Vázquez EM et al (2021) Immunotherapy in nonsmall-cell lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol, Immunother CII 70(5):1177–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck SF (1960) A method of estimation of missing values in multivariate data suitable for use with an electronic computer. J Roy Stat Soc 22(2):302–306 [Google Scholar]
- Chen Y et al (2023) Pan-immune-inflammation and its dynamics: predictors of survival and immune-related adverse events in patients with advanced NSCLC receiving immunotherapy. BMC Cancer 23(1):944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creators Davidson-Pilon C (2022) Lifelines, survival analysis in Python. https://doi.org/10.5281/zenodo.7329096
- Crosbie PAJ et al (2013) Prognostic and predictive biomarkers in early stage NSCLC: CTCs and serum/plasma markers. Transl Lung Cancer Res 2(5):382–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallolio FG et al (2020) CEA and CYFRA 21–1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Therap Adv Med Oncol 12:1758835920952994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll JJ, Rixe O (2009) Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J 15(5):401–405 [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247 [DOI] [PubMed] [Google Scholar]
- Essink A, Korse T, van den Heuvel M (2016) 157P: Serum tumor markers and the response to immunotherapy in advanced non-small cell lung carcinoma. J Thorac Oncol 11(4 Supplement):S126 [Google Scholar]
- Farina B et al (2023) Integration of longitudinal deep-radiomics and clinical data improves the prediction of durable benefits to anti-PD-1/PD-L1 immunotherapy in advanced NSCLC patients. J Transl Med 21(1):174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier L et al (2021) Twenty years On: RECIST as a biomarker of response in solid tumours an EORTC imaging group—ESOI joint paper. Front Oncol 11:800547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst MA et al (1998) Support vector machines. IEEE Intell Syst Their Appl 13(4):18–28 [Google Scholar]
- Hellmann MD et al (2020) Circulating tumor DNA analysis to assess risk of progression after long-term response to PD-(L) 1 blockade in NSCLCctDNA analysis in long-term responders to PD-(L) 1 blockade. Clin Cancer Res 26(12):2849–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T et al (2022) Validating ORR and PFS as surrogate endpoints in phase II and III clinical trials for NSCLC patients: difference exists in the strength of surrogacy in various trial settings. BMC Cancer 22(1):1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannessi A et al (2021) RECIST 1.1 and lesion selection: how to deal with ambiguity at baseline? Insights Imaging 12(1):36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen S et al (2019) Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open 4(4):e000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M et al (2020) Clinical parameters for predicting the survival in patients with squamous and non-squamous-cell NSCLC receiving PD-1 inhibitor therapy. Pathol Oncol Res POR 26(1):327–333 [DOI] [PubMed] [Google Scholar]
- Jiang T, Zeng Q, He J (2023) Do alkaline phosphatases have great potential in the diagnosis, prognosis, and treatment of tumors? Transl Cancer Res 12(10):2932–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian C et al (2022) Real-world data prognostic model of overall survival in patients with advanced NSCLC receiving anti-PD-1/PD-L1 immune checkpoint inhibitors as second-line monotherapy. Cancer Rep 5(10):e1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar A et al (2019) Interobserver variation in response evaluation criteria in solid tumors 1.1. Acad Radiol 26(4):489–501 [DOI] [PubMed] [Google Scholar]
- Klümper N et al (2022) C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer. 10.1136/jitc-2021-004024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D et al (2019) Early serum tumor marker dynamics predict progression-free and overall survival in single PD-1/PD-L1 inhibitor treated advanced NSCLC-a retrospective cohort study. Lung Cancer 134:59–65 [DOI] [PubMed] [Google Scholar]
- Li H et al (2019) Circulating tumor DNA detection: a potential tool for colorectal cancer management. Oncol Lett 17(2):1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y et al (2022) Machine learning models for identifying predictors of clinical outcomes with first-line immune checkpoint inhibitor therapy in advanced non-small cell lung cancer. Sci Rep 12(1):17670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg SM et al (2020) From local explanations to global understanding with explainable AI for trees. Nature Mach Intell 2(1):56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menekse S, Kut E, Almuradova E (2023) Elevated serum lactate dehydrogenase to albumin ratio is a useful poor prognostic predictor of nivolumab in patients with non-small cell lung cancer. Eur Rev Med Pharmacol Sci 27(5 Suppl):86–94 [DOI] [PubMed] [Google Scholar]
- Morse B et al (2019) Pearls and pitfalls of response evaluation criteria in solid tumors (RECIST) v1.1 non-target lesion assessment. Abdominal Radiol (new York) 44(2):766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AN et al (2022) Prediction of lung cancer immunotherapy response via machine learning analysis of immune cell lineage and surface markers. Cancer Biomark: Sect A Dis Mark 34(4):681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M et al (2021) Validation of a clinical blood-based decision aid to guide immunotherapy treatment in patients with non-small cell lung cancer. Tumour Biol J Int Soc Oncodev Biol Med 43(1):115–127 [DOI] [PubMed] [Google Scholar]
- Mushti SL, Mulkey F, Sridhara R (2018) Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin Cancer Res 24(10):2268–2275 [DOI] [PubMed] [Google Scholar]
- Pavan A et al (2019) Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist 24(8):1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830 [Google Scholar]
- Peng L et al (2020) Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother CII 69(9):1813–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl JM et al (2020) C-reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non-small cell lung cancer: a bi-center study. Cancers. 10.3390/cancers12082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochigneux P et al (2022) Mass cytometry reveals classical monocytes, NK cells, and ICOS+ CD4+ T cells associated with pembrolizumab efficacy in patients with lung cancer. Clin Cancer Res 28(23):5136–5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid AM et al (2021) Radiologists and clinical trials: part 1 the truth about reader disagreements. Ther Innov Regul Sci 55(6):1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour L et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 10.1016/s1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shameer K et al (2021) Correlation between early endpoints and overall survival in non-small-cell lung cancer: a trial-level meta-analysis. Front Oncol 11:672916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki J et al (2018) Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 13(1):97–105 [DOI] [PubMed] [Google Scholar]
- Tovoli F et al (2018) Inter-operator variability and source of errors in tumour response assessment for hepatocellular carcinoma treated with sorafenib. Eur Radiol 28(9):3611–3620 [DOI] [PubMed] [Google Scholar]
- Trebeschi S, Bodalal Z, Boellaard TN, Tareco Bucho TM, Drago SG, Kurilova I, Calin-Vainak AM, Delli Pizzi A, Muller M, Hummelink K, Hartemink KJ, Nguyen-Kim TDL, Smit EF, Aerts HJWL, Beets-Tan RGH (2021) Prognostic value of deep learning-mediated treatment monitoring in lung cancer patients receiving immunotherapy. Front Oncol 11:609054. 10.3389/fonc.2021.609054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K (2011) Mice: multivariate imputation by chained equations in R. J Stat Softw 45:1–67 [Google Scholar]
- van Delft FA et al (2022) Modeling strategies to analyse longitudinal biomarker data: an illustration on predicting immunotherapy non-response in non-small cell lung cancer. Heliyon 8(10):e10932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q-S, Liang Y-Z (2001) Monte Carlo cross validation. Chemom Intell Lab Syst 56(1):1–11 [Google Scholar]
- Yang T et al (2023) Pretreatment levels of serum alkaline phosphatase are associated with the prognosis of patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Oncol Lett 25(4):154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J et al (2020) Relationship between progression-free survival, objective response rate, and overall survival in clinical trials of PD-1/PD-L1 immune checkpoint blockade: a meta-analysis. Clin Pharmacol Ther 108(6):1274–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH et al (2016) Observer variability in RECIST-based tumour burden measurements: a meta-analysis. Eur J Cancer 53:5–15 [DOI] [PubMed] [Google Scholar]
- Zhang Z et al (2018) Time-varying covariates and coefficients in COX regression models. Ann Transl Med 6(7):121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q et al (2022) The predictive value of pretreatment lactate dehydrogenase and derived neutrophil-to-lymphocyte ratio in advanced non-small cell lung cancer patients treated with PD-1/PD-L1 inhibitors: a meta-analysis. Front Oncol 12:791496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-G et al (2022) Machine learning based on blood test biomarkers predicts fast progression in advanced NSCLC patients treated with immunotherapy. 10.2139/ssrn.4214527 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, S.T., upon reasonable request.