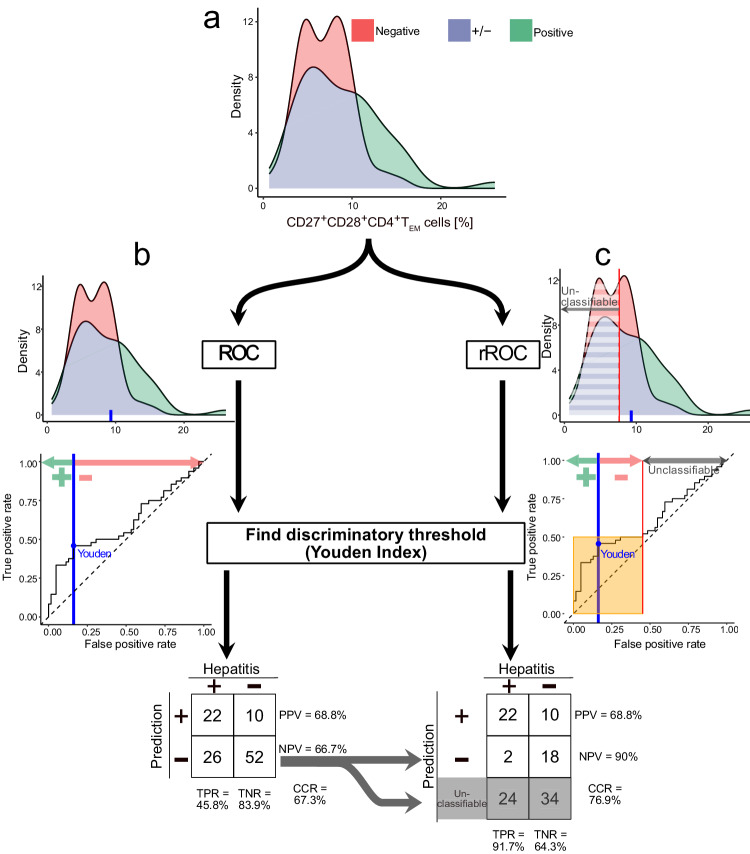
Fig. 7. Clinical interpretation of restricted biomarkers in predicting disease.
Our method of dataset restriction leads to counterintuitive clinical interpretations of biomarker values. This is illustrated by our discovery of CD27+ CD28+ CD4+ TEM cells as a univariate biomarker of hepatitis risk after immunotherapy. Here, we illustrate the conventional evaluation of biomarker performance across all samples with evaluation of biomarker performance in a restricted dataset. a Densities of CD27+ CD28+ CD4+ TEM cells in all samples from patients with metastatic melanoma who developed hepatitis (n = 48) or did not (n = 62) after starting Ipi-Nivo therapy. b Following the classical approach of determining a classification cutoff for CD27+ CD28+ CD4+ TEM frequency relative to CD4+ T cells using the Youden Index, we predict hepatitis if > 9.62% and then assess the correct classification rate (CCR), negative predictive value (NPV), positive predictive value (PPV), sensitivity (or true positive rate, TPR) and specificity (or true negative rate, TNR) for all samples. c Our restriction method is predicated on there being a range of values over which a biomarker provides no discriminatory information. Optimally restricting CD27+ CD28+ CD4+ TEM cell values leads us to discard 58 of 110 samples as “unclassifiable.” For the remaining 42 samples where CD27+ CD28+ CD4+ TEM frequency relative to CD4+ T cells > 7.62%, we determine a classification cutoff using the Youden Index, again predicting hepatitis if > 9.62%. Accordingly, we obtain a confusion table with CCR = 76.9%, specificity = 64.3% sensitivity = 91.7%, PPV = 68.8% and NPV = 90% across the classifiable samples.