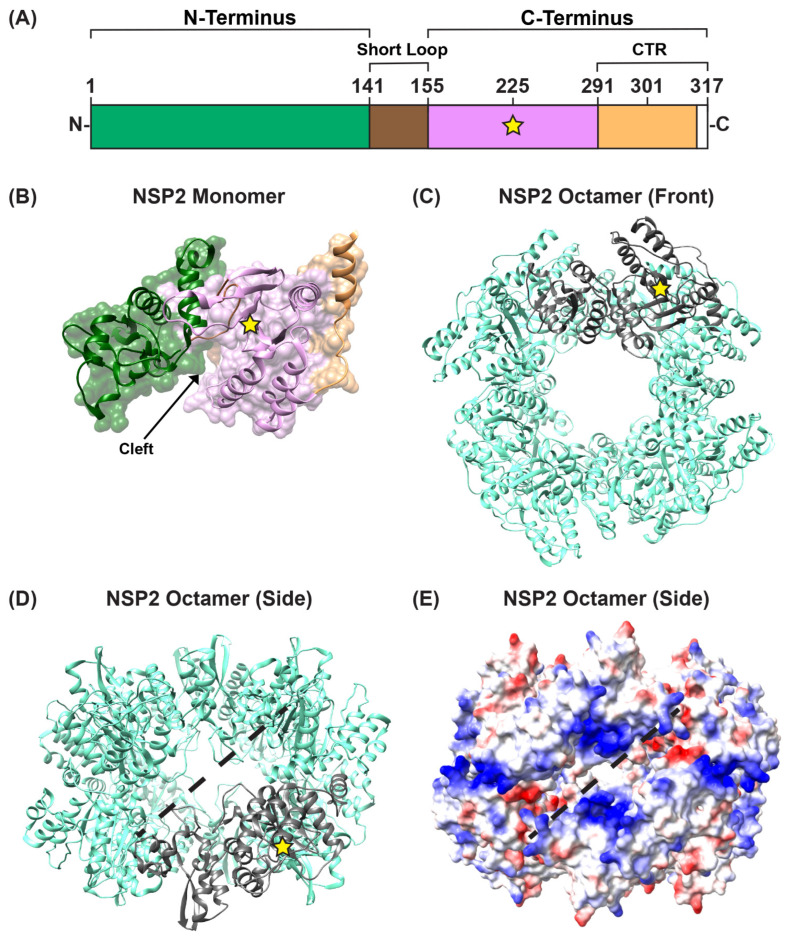
Figure 3.
NSP2 Structure. (A) Linear schematic of strain SA11 NSP2 (317 amino acids in length). The protein is comprised of two domains: an N-terminal (green) and a C-terminal domain (pink), separated by a short loop (brown). The extreme C-terminal region (CTR; residues 291–317) is represented in orange; however, CTR residues 314 to 317 are unstructured (white). A yellow star represents the catalytic site H225. (B) SA11 NSP2 monomer (PDB no. 1L9V) is colored as in panel (A) and is shown in both surface and ribbon representation. An arrow indicates the electropositive cleft, and a yellow star represents the catalytic site H225. (C) SA11 NSP2 octamer structure (PDB no. 1L9V) is shown in ribbon representation (cyan), which a single monomer highlighted in gray. (D) SA11 NSP2 octamer from panel (C) is flipped “forward” 90 degrees to show the side view along the two-fold axis. The electropositive groove that comprises RNA/NSP5 binding site is shown as a dashed line. (E) SA11 NSP2 octameric structure from panel (D) is shown in electrostatic surface representation. Red indicates negative charge, while blue indicates positive charge.