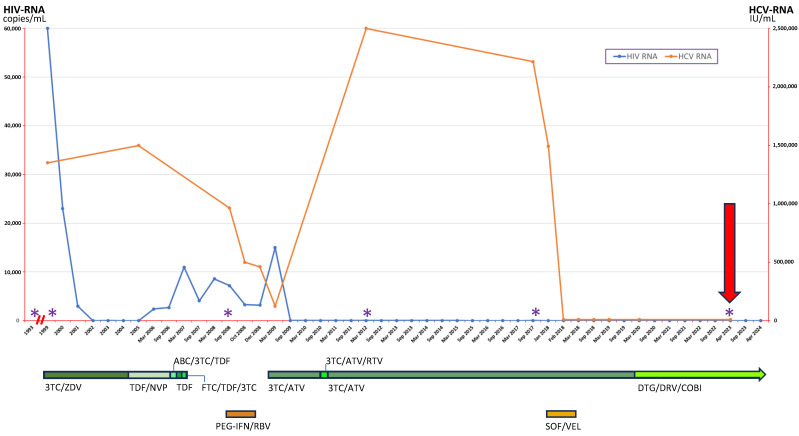
Figure 1.
Timeline with relevant data from the clinical history of the patient, which resulted anti-HD positive. The upper part of the figure depicts data with respect to HIV-RNA (blue line) and HCV-RNA (orange line) trends; the purple asterisks represent the serialized controls of virological tests for HBV (HBV-DNA and complete serology, which were always completely negative). The lower part of the figure depicts the treatment regimens she underwent for both HIV (in green) and HCV (in orange) infections. The arrow refers to the time when the patient was sampled for the present study (April 2023), as reported in Table 1. Abbreviations: abacavir (ABC); atazanavir (ATV); cobicistat (COBI); darunavir (DRV); dolutegravir (DTG); emtricitabine (FTC); hepatitis B virus (HBV); hepatitis C virus (HCV); human immunodeficiency virus (HIV); lamivudine (3TC); nevirapine (NVP); pegylated interferon (PEG-IFN); ribavirin (RBV); ritonavir (RTV); sofosbuvir (SOF); tenofovir disoproxil fumarate (TDF); velpatasvir (VEL); zidovudine (ZDV).