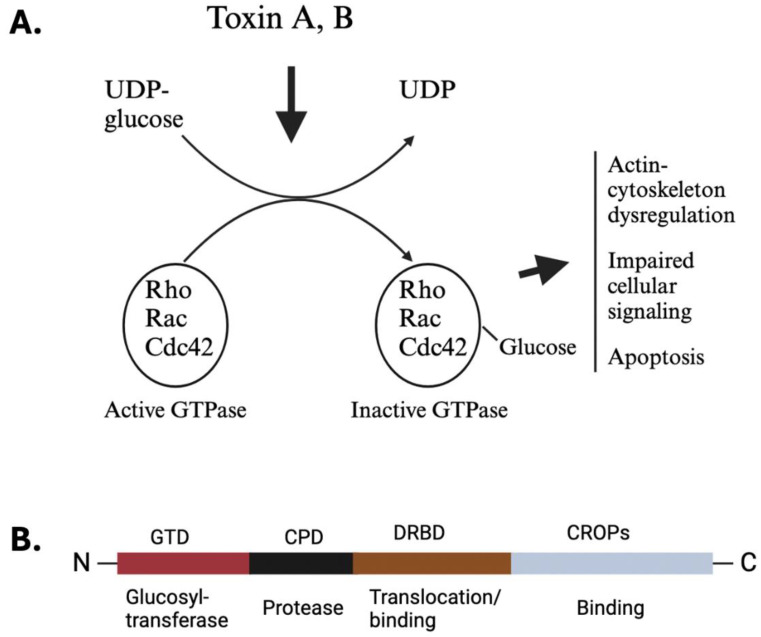
Figure 1.
The deleterious effect of C. difficile toxins on host cells and their general structure. (A) The acidic environment inside the endosome triggers the release of the enzymatic glucosyltransferase domain situated at the N-terminus of toxins A and B. Once released, this glucosyltransferase enzyme inactivates small GTPases by adding glucose molecules. This disruption of GTPase function by the toxins leads to several deleterious cellular effects, such as disorganization of the actin cytoskeleton, weakening of tight junctions between cells, apoptosis, acute inflammation, and changes in cellular signaling pathways. (B) The schematics depict the basic layout of C. difficile’s toxins A and B, highlighting the four functional domains of TcdB. Starting at the N-terminus, there is the glucosyltransferase domain (GTD, red), followed by the inherent cysteine protease (black), the delivery and binding domain (DRBD, brown), and finally, at the C-terminus, the CROP domain (gray), which also plays a role in binding.