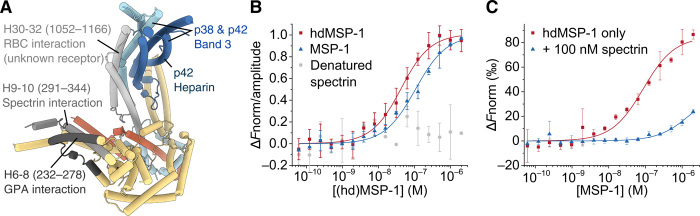
Fig. 8. Interaction surfaces.
(A) Various regions of MSP-1 that have been shown to interact with erythrocyte targets (15, 23–25) are indicated. Note that the MSP-1 regions were identified in studies using MSP-1 fragments, and other MSP-1 areas might also interact with the named targets. See main text for details. (B) MSP-1 was shown to bind spectrin using MST. KD values of 31 ± 5 nM (n = 4, red squares) and 100 ± 10 nM (n = 2, blue triangles) were found for the hdMSP-1–spectrin and MSP-1–spectrin complexes, respectively. No significant binding of MSP-1 to heat-denatured spectrin was observed (gray circles). (C) MST analysis of the dimerization affinity for hdMSP-1 in the absence (red squares) and presence (blue triangles) of 100 nM unlabeled spectrin yielded KD values of 80 ± 20 nM (n = 2) and 1.7 ± 0.9 μM (n = 2), respectively. Error bars represent SD.