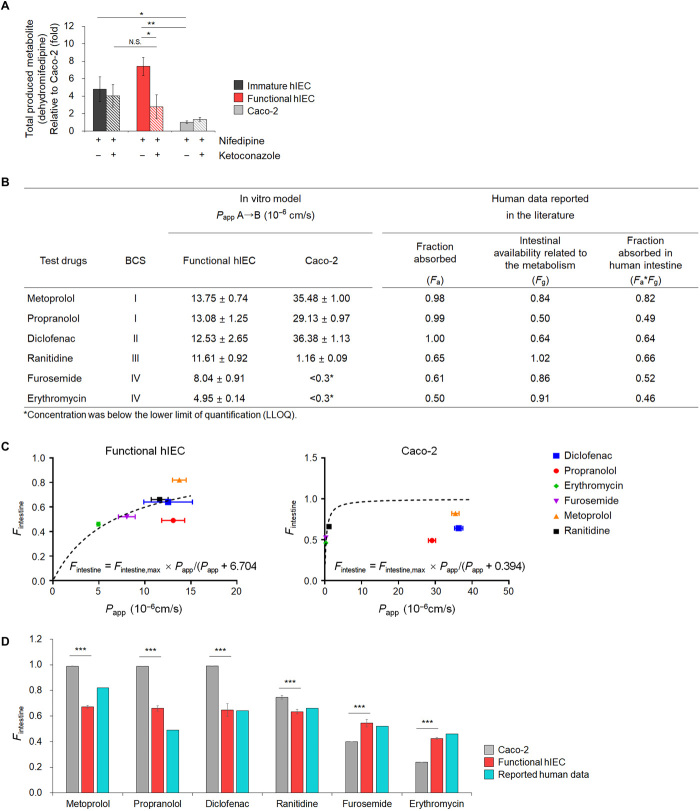
Fig. 7. Drug metabolizing activity of CYP3A4 using nifedipine and prediction of Fa × Fg in humans based on functional hIEC monolayers.
(A) Concentration of dehydro-nifedipine following 2 hours incubation in the absence or presence of ketoconazole, a specific CYP3A4 inhibitor, in immature hIECs, functional hIECs, and Caco-2 cells. Data represent means ± SD (n = 3). *P < 0.05 and **P < 0.01 by two-tailed t test. (B) Apical-to-basolateral permeability in functional hIECs and Caco-2 monolayers for six model drugs. Apparent permeability values (Papp) represent means ± SD (n = 3). Values for fraction absorbed by the human intestine (Fintestine) were obtained from literature. (C) The hyperbolic model fitted between the in vivo human Fintestine and observed Papp for model drugs in functional hIECs or Caco-2 cells. The dotted lines represent a fitted relationship curve, which was used to estimate Fintestine using the observed Papp. The equation for model structure was Fintestine = Fintestine,max × Papp(10−6 cm/s)/[Papp (10−6 cm/s) + Kd]; Kd represents the Papp at which Fintestine is 50% of Fintestine,max. (D) Comparison of predicted fraction absorbed by the human intestine [Fintestine; Fa (the fraction of orally administered drugs absorbed from the intestine) × Fg (the fraction of intestinal availability related to the metabolism)] of six model compounds in functional hIEC and Caco-2 cell system. Fintestine was calculated using the established model equation. The data represent means ± SD (n = 3). ***P < 0.001 by two-tailed t test.