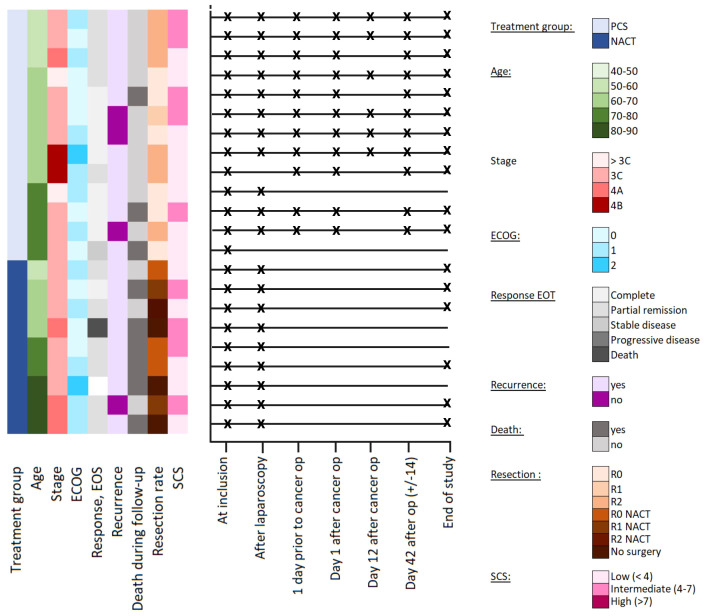
Figure 1.
Overview and timeline of the patients. The crosses indicate time points for collecting serum samples; the absence of a cross on the line indicates that serum samples were not obtained at this time point. ECOG, Eastern Cooperative Oncology Group Score; EOS, End of study; NACT, neoadjuvant chemotherapy; PCS, primary cytoreductive surgery; Post-surgery: samples taken 1 day after primary cytoreductive surgery; Pre-chemo: Samples taken day 42 after surgery (+/- 14 days); Pre-surgery: samples taken 1 day prior to primary cytoreductive surgery; R0: Complete cytoreductive surgery; R1: Optimal cytoreductive surgery, residual tumor size ≤1 cm; R2: suboptimal cytoreductive surgery; residual tumor size >1; SCS, surgical complexity score.