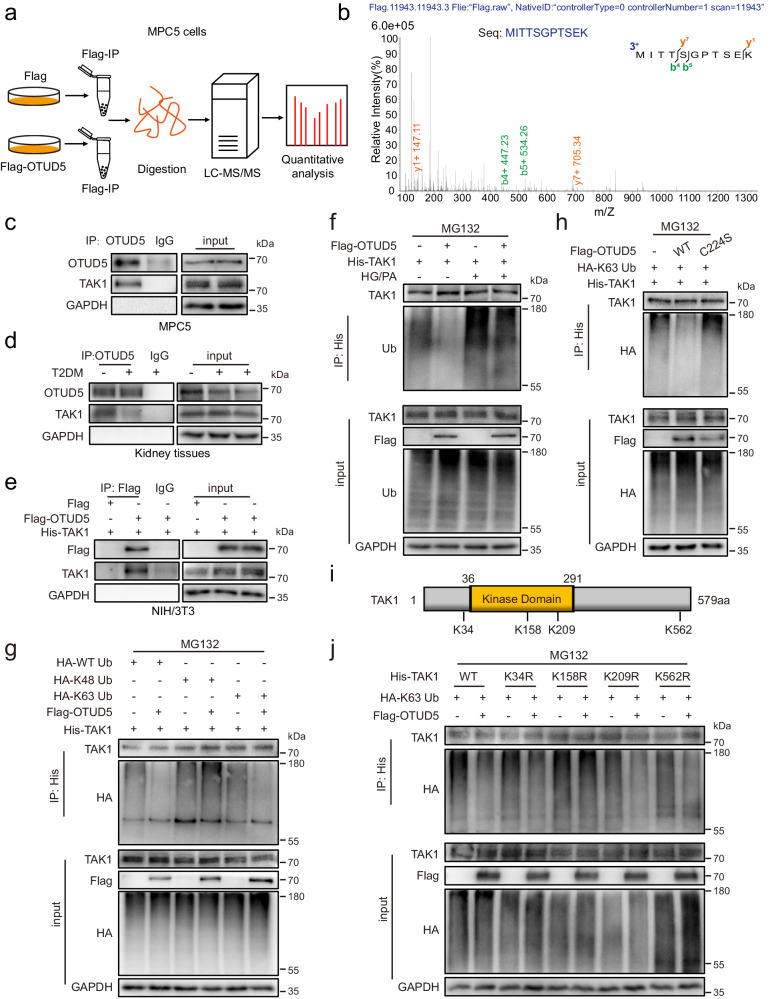
Fig. 4. Identification of TAK1 as a potential substrate protein of OTUD5.
a Schematic illustration of a quantitative proteomic screen to identify proteins binding to OTUD5. b Mass spectrometry/mass spectrometry (MS/MS) spectrum of the peptide MITTSGPTSEK from TAK1. Co-immunoprecipitation (Co-IP) of OTUD5 and TAK1 in MPC5 cells (c) and kidney tissues (d). Endogenous OTUD5 was immunoprecipitated. (n = 3 independent experiments). e Co-IP of OTUD5 in NIH/3T3 co-transfected with Flag-OTUD5 and His-TAK1 plasmids. Exogenous OTUD5 was immunoprecipitated using an anti-Flag antibody. (n = 3 independent experiments). f His-TAK1 and Flag-OTUD5 were transfected into MPC5 cells with or without HG/PA treatment and then subjected to 10 μM MG132 for 6 h. Ubiquitinated TAK1 was detected by immunoblotting using an anti-ubiquitin antibody. (n = 3 independent experiments). g His-TAK1, HA-WT Ub, HA-K48 Ub, and HA-K63 Ub were transfected into NIH/3T3 together with Flag-OTUD5 and then subjected to 10 μM MG132 for 6 h. Ubiquitinated TAK1 was detected by immunoblotting using an anti-HA antibody. (n = 3 independent experiments). h His-TAK1 and HA-K63 Ub were transfected into NIH/3T3 together with Flag-OTUD5 (WT or C224S) and then subjected to 10 μM MG132 for 6 h. Ubiquitinated TAK1 was detected by immunoblotting using an anti-HA antibody. (n = 3 independent experiments). i Schematic illustration of the construct for mutating the ubiquitinated lysine residue of TAK1. j His-TAK1 (WT, K34R, K158R, K209R or K562R) and HA-WT Ub were transfected into NIH/3T3 together with Flag-OTUD5 and then subjected to 10 μM MG132 for 6 h. Ubiquitinated TAK1 was detected by immunoblotting using an anti-HA antibody. (n = 3 independent experiments).