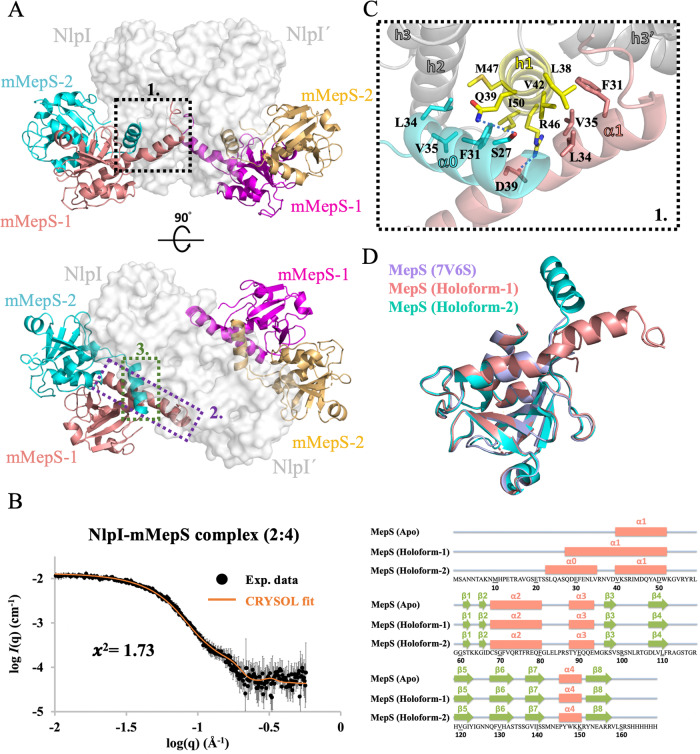
Fig. 3. Structural insights into the NlpI-mMepS complex by crystal structure and SAXS.
A Two orthogonal views of the NlpI homodimer complexed with mMepS are depicted with the indicated location of the interaction sites, highlighting the binding interfaces between the proteins. The detailed interactions highlighted in dashed boxes 1 to 3 are further described in Fig. 3C and Supplementary Fig. 8. The surface of the NlpI dimer is rendered in grey, while the bound mMepS proteins are represented in cartoon form using colors of salmon, cyan, magenta, and golden. This color scheme is consistently used throughout the entire document. B SAXS analysis was conducted on the NlpI-mMepS complex, and the results were compared with the theoretical SAXS curve calculated from the crystal structure of the complex. The superimposition of the experimental size exclusion chromatography coupled with small-angle X-ray scattering (SEC-SAXS) profile in black and the theoretical SAXS curve in orange showed alignment, with a χ2 value of 1.73, confirming the presence of the hexameric structure in solution. The error bars represent the root-mean-square deviations of the measured scattering intensity at each scattering vector (q) value. Source data are provided as a Source Data file. C The interactions between NlpI and mMepS proteins involve specific hydrophobic contacts. The hydrophobic side chains of L24, F31, L34, V35, and V38 in the extended alpha-1 region of mMepS-1 form hydrophobic contacts with residues L38, V42, I43, and A45 of NlpI h1, as well as residues R78’-L80’, Y105’, and Q108’ located at NlpI’ h3’-h4’. D Secondary structure comparison of mMepS in the context of apo state and NlpI-bound states. The apo form of mMepS features an intrinsically disordered N-terminal preceding the core domain, as characterized by our NMR chemical shift assignments (BMRB ID 51949). The α helices are depicted as salmon rectangles, while the β strands are indicated by green arrows. The secondary structural elements of NlpI-bound mMepS and the apo form of mMepS (PDB ID 7V6S) are shown above the protein sequence, with the α helices depicted as salmon rectangles, and green arrows indicating the β strands.