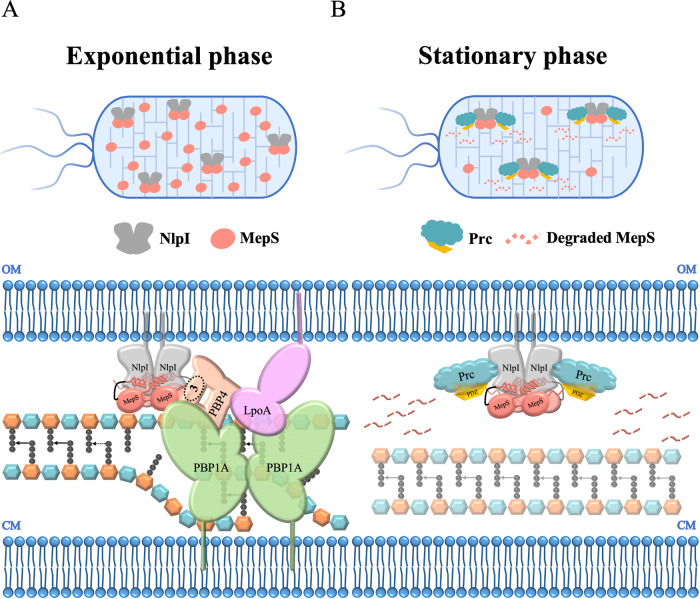
Fig. 7. Model illustrating the scaffolding function of NlpI for coordinating PG endopeptidases and PG synthetic machinery.
A A simplified model for the potential role of NlpI in coordinating the PG multi-enzyme complex, involving LpoA (a synthesis regulator), PBP1A (a bifunctional synthase), NlpI, and various endopeptidases during the exponential growth phase. This model involves the outer membrane (OM) lipoprotein NlpI, which acts as an adaptor protein to scaffold different endopeptidases into a trimeric complex. Specifically, NlpI forms a complex with MepS and PBP4, and potentially with PBP739. Afterwards, the PBP4 (or PBP7), as a linking partner, could further recruit the PG synthesis machinery, PBP1A/LpoA to facilitate the formation of PG multi-enzyme complexes (PBP1A-MepS-NlpI-PBP4-LpoA or PBP1A-MepS-NlpI-PBP7-LpoA). PBP4 is found to interact with NlpI through its domain 3, as encircled and labeled in the model. Some stem peptides are removed for clarity. This proposed model provides insights into the potential mechanisms by which NlpI coordinates the activities of various enzymes involved in peptidoglycan synthesis. B The proposed model illustrates the direct control of the protein levels of endopeptidase MepS by the Prc-NlpI proteolytic system, which is crucial for maintaining cell envelope integrity during the stationary phase. As bacterial cells transition into the stationary phase, MepS-bound NlpI recruits the E. coli periplasmic PDZ-protease Prc, leading to rapid degradation of MepS. This regulatory mechanism plays a vital role in ensuring cell wall stability under stationary phase conditions.