Abstract
BACKGROUND
Lung cancer bone metastasis (LCBM) is a disease with a poor prognosis, high risk and large patient population. Although considerable scientific output has accumulated on LCBM, problems have emerged, such as confusing research structures.
AIM
To organize the research frontiers and body of knowledge of the studies on LCBM from the last 22 years according to their basic research and translation, clinical treatment, and clinical diagnosis to provide a reference for the development of new LCBM clinical and basic research.
METHODS
We used tools, including R, VOSviewer and CiteSpace software, to measure and visualize the keywords and other metrics of 1903 articles from the Web of Science Core Collection. We also performed enrichment and protein-protein interaction analyses of gene expression datasets from LCBM cases worldwide.
RESULTS
Research on LCBM has received extensive attention from scholars worldwide over the last 20 years. Targeted therapies and immunotherapies have evolved into the mainstream basic and clinical research directions. The basic aspects of drug resistance mechanisms and parathyroid hormone-related protein may provide new ideas for mechanistic study and improvements in LCBM prognosis. The produced molecular map showed that ribosomes and focal adhesion are possible pathways that promote LCBM occurrence.
CONCLUSION
Novel therapies for LCBM face animal testing and drug resistance issues. Future focus should centre on advancing clinical therapies and researching drug resistance mechanisms and ribosome-related pathways.
Keywords: Lung cancer, Bone metastasis, Bibliometrics, Targeted therapy, Immunotherapy, Enrichment analysis
Core Tip: A comprehensive analysis of 1903 publications from 2000 to 2022 was conducted to identify trends in lung cancer bone metastasis (LCBM) research. The literature on LCBM was systematically organized and analysed according to the changing trends in the knowledge volume, distribution of countries/regions, evolution of research topics and co-occurrence of keywords. The results show that immunotherapy and targeted therapy are new research hotspots and ribosome-related genes play an important role in LCBM. This study also preliminarily explored the potential molecular biological mechanisms of LCBM, which mainly involved ribosome and focal adhesion pathways.
INTRODUCTION
Currently, lung cancer (LC) bone metastasis (LCBM) is an incurable disease[1]. Patients with LCBM often experience bone and joint pain, pathological fractures and neuropathic pain, which all seriously affect their prognoses and quality of life[2,3]. For many years, LC has had high incidence and mortality rates and the difficulties in early diagnosis mean that many patients have advanced LC at the time of diagnosis[4,5]. The size of the patient population with LCBM is not optimistic as more than 30% of the patients with advanced LC may develop BM[6]. Considering these facts, LCBM can be understood as a life-threatening disease in terms of both the clinical data and symptoms, and it thus requires further study.
Over the past 20 years, a wide range of researchers have gradually started to concentrate on the study of LCBM, and a considerable amount of scientific output has accumulated. However, problems, such as confusing research structures, have arisen. Moreover, no comprehensive or objective metrological studies have been reported on topics such as the tracking or evaluation of the research dynamics of LCBM research. Therefore, while dealing with rapid changes in the study of LCBM, researchers not only face difficulty in quickly grasping these developments, they must also waste additional effort as a result[7,8]. For example, they must also waste additional effort sifting through studies to find relevant data. Bibliometrics, which have the advantage of objectively analysing a large amount of literature on multiple levels, can help solve these problems and have been used extensively in the field of medical research[9].
Therefore, we performed a bibliometric analysis of metrics such as the keywords in articles on LCBM from the Web of Science Core Collection (WOSCC). In addition, we conducted a preliminary investigation of the potential molecular biological mechanisms in LCBM. We hope that this study will help researchers interested in LCBM quickly and accurately grasp the research dynamics involved and provide insights into the global developments in LCBM research.
MATERIALS AND METHODS
Collection of data from articles on LCBM
The data from the articles on LCBM were obtained from the WOSCC (https://www.webofscience.com/). The search formula used in this study was as follows: TS = ((“osteolytic metastas*”) OR (“metastatic tumor of bone*”) OR (“bone metastas*”) OR (“osteolytic metastas*”) OR (“osteoblastic metastas*”) OR (“skeletal metastas*”) OR (“bone marrow metastas*”) OR (“metastatic carcinoma of bone marrow”) OR (“metastatic bone disease”)) AND ((“lung cancer”) OR (“cancer of the lung”) OR(“cancer of the lung*”) OR (“lung *carcinoma”) OR (“*carcinoma of the lung*”)).
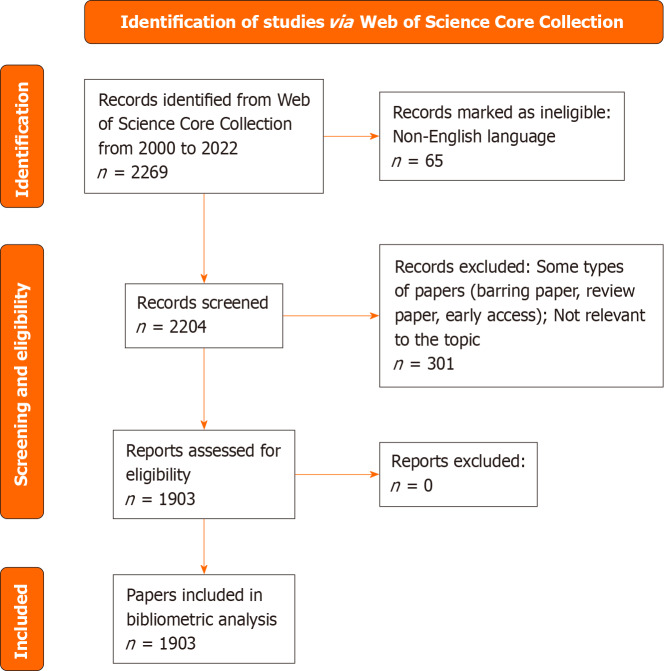
The inclusion and exclusion criteria for the LCBM articles in this study were as follows: (1) To avoid the effects of too many changes in the data due to updates or limitations of the WOSCC, only articles published between 2000 and 2022 were included; (2) To ensure the rigor of the analysis results, only three types of articles were included: Articles, review articles and early-access articles; and (3) Owing to the limitations of the software, only articles written in English were included. In the end, we included 1903 articles (Figure 1).
Figure 1.
Flowchart of data collection from articles on lung cancer bone metastasis.
All the data for this study were downloaded from the WOSCC in the BibTeX format on January 9, 2023, and the records were “Full Record and Cited Reference”. The data collection was performed by two authors. Disagreements between the two authors during this process were discussed in depth and eventually resolved by both authors along with the other authors.
Bibliometric analysis of the data from the articles on LCBM
We used the powerful features of R software (4.2.2), such as advanced statistical calculations, visualization and complete bibliometric analysis, to produce topic evolution maps, keyword time heat maps and other materials. In this study, we also took advantage of VOSviewer software (1.6.18), which can handle large amounts of data and provide strong graphical presentation capabilities for producing keyword clustering maps and other visualisation tools. In addition, we used CiteSpace software (6.1.R6 basic), which can detect hotspot sensitively, and produced keyword burst detection maps.
Exploration of the molecular mechanisms of LCBM
We screened the GSE175601 dataset in the Gene Expression Omnibus, The Cancer Genome Atlas, Sequence Read Archive and ArrayExpress databases based on the criterion that the data should be from Homo sapiens tissues with both LC and LCBM tissue data and downloaded the dataset. We then used R software to extract the expression matrix from the dataset and screen the differentially expressed genes (DEGs) of LCBM. The criteria for screening the DEGs were |log fold change| > 1 and P values < 0.05. We then performed Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes and Reactome enrichment analyses of the screened DEGs as a preliminary analysis of the potential molecular biological mechanisms of LCBM. In addition, we used STING (v11.5) and Cytoscape (v3.9.1) to construct protein-protein interaction (PPI) network maps to further analyse the mechanisms involved in LCBM.
RESULTS
Changes in the volume and distribution of knowledge of LCBM
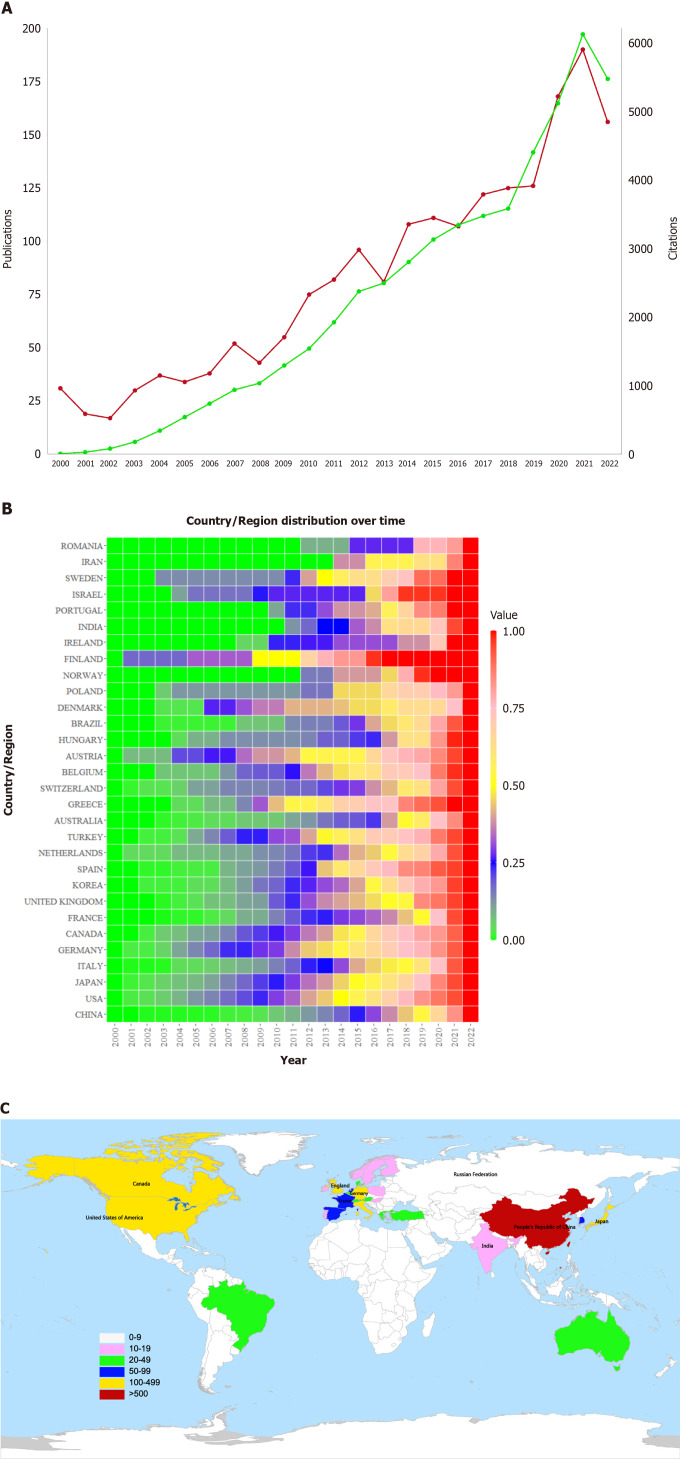
Figure 2A reflects the overall trend of the year-on-year increase in the number of publications and citations since 2000, when 31 papers were published with 10 citations. The volume of knowledge skyrocketed during the last 3 years, with the number of publications exceeding a quarter of the volume of the articles included in this study. The number of citations was no fewer than 10 across 77 articles. Among all of the countries where these articles were published, China had the highest number of publications (602), accounting for approximately one-third of the articles included in this study (Table 1). In addition, other countries that were major contributors to LCBM research include the United States, Japan, and Italy. As shown in Figure 2B and C, developing countries, such as China, Iran, and India, have seen a surge in their number of LCBM-related publications in recent years.
Figure 2.
Annual change and distribution map of lung cancer bone metastasis knowledge volume. A: Annual publications and citations of articles on lung cancer bone metastasis (LCBM) from 2000 to 2022, the red line corresponds to “publications”; the green line corresponds to “citations”; B: Thermal diagram of the time distribution of national/regional articles, the values represent the ratio of the total number of papers published in a country from 2000 to a certain year to the total number of papers published in a country; C: Analysis chart of articles on LCBM at the country/region level.
Table 1.
Number of articles on lung cancer bone metastasis that were published in countries/regions
|
Country/region
|
Number of publications
|
| China | 602 |
| United States | 446 |
| Japan | 238 |
| Italy | 123 |
| Canada | 122 |
| Germany | 121 |
| United Kingdom | 109 |
| Republic of Korea | 70 |
| France | 68 |
| Spain | 60 |
| Netherlands | 53 |
| Turkey | 46 |
| Australia | 42 |
| Switzerland | 41 |
| Belgium | 33 |
| Austria | 32 |
| Greece | 31 |
| Brazil | 25 |
| Denmark | 21 |
| Hungary | 16 |
Thematic changes in LCBM research
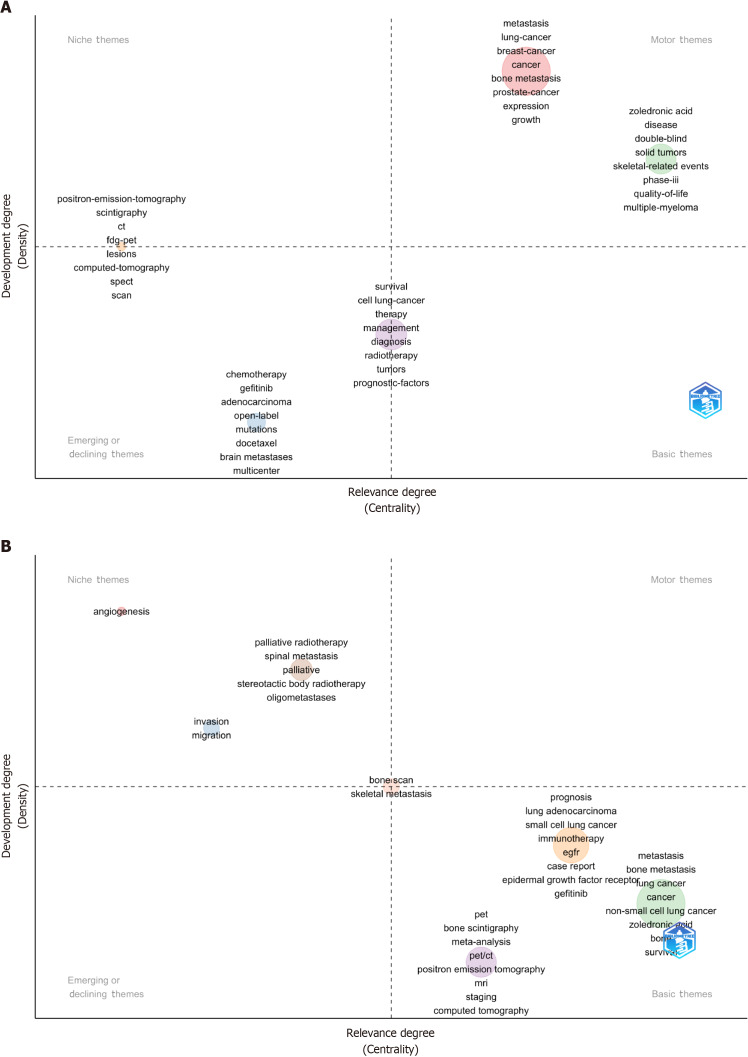
Thematic strategic coordinate map for LCBM: We analysed the keywords plus (ID) and authors’ keywords (DE) in the LCBM articles (Supplementary Tables 1 and 2, Supplementary Figure 1) and depicted the LCBM hotspot changes using a theme strategy coordinate map (Figure 3). Among the themes identified, zoledronic acid, quality of life and skeletal-related events are the mature and important topics. Palliative, palliative radiotherapy and stereotactic body radiotherapy are mature but currently unimportant topics. Immunotherapy, meta-analysis and epidermal growth factor receptor (EGFR) are immature but important topics, and chemotherapy and docetaxel are marginal topics.
Figure 3.
Theme strategic coordinate map. A: Keywords plus; B: Authors’ keywords.
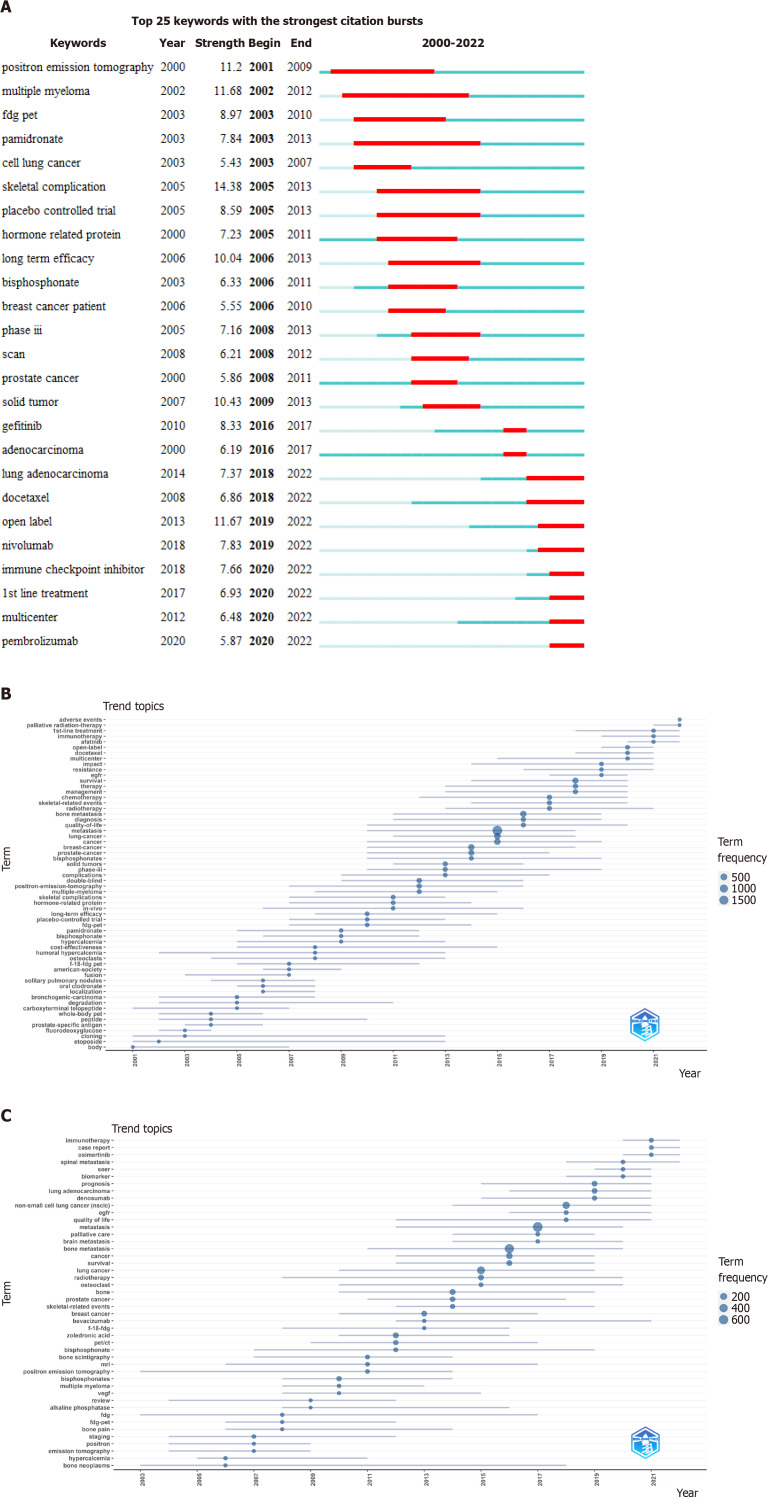
Evolution of the subject terms related to LCBM: We further analysed the changes in the hot topics to capture the knowledge changes in the research on LCBM (Figures 4 and 5). Among the hot topics, immunotherapy, osimertinib, afatinib, EGFR, palliative radiotherapy and SEER have become new focuses of attention, while brain metastasis, lung adenocarcinoma, non-small cell LC and other similar topics have long been the focus of LCBM research.
Figure 4.
Time distribution chart of popular keywords. A: Burst detection graph of all keywords in articles on lung cancer bone metastasis (LCBM); B: Topic trend graph drawn based on keywords plus of the articles on LCBM; C: Topic trend graph drawn based on the authors’ keywords in the articles on LCBM.
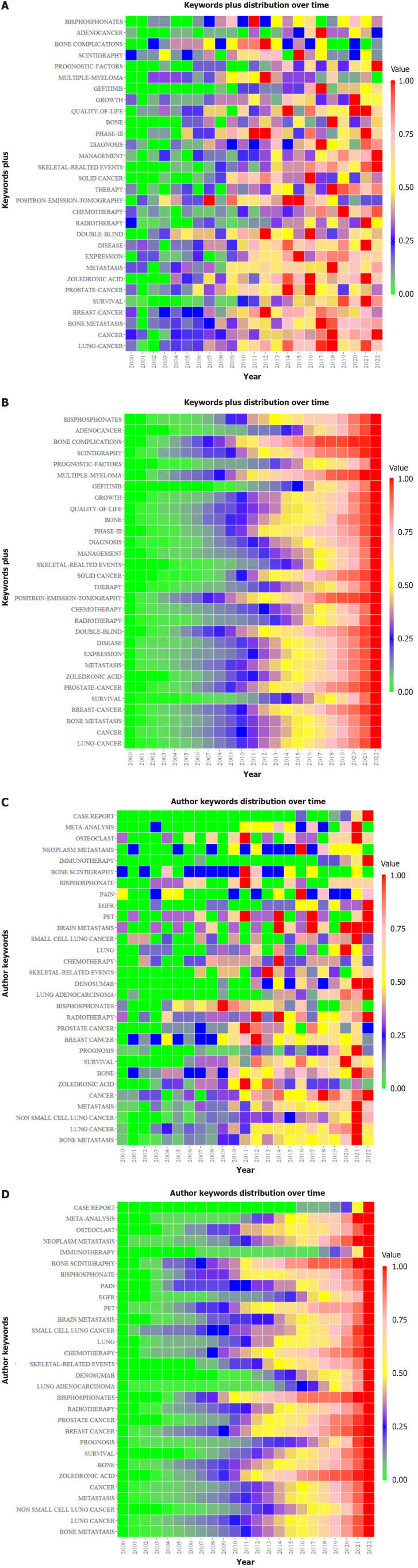
Figure 5.
Keyword time heat map. A and B: Keywords plus; C and D: Authors’ keywords. The A and C values represent the ratio of the frequency of a keyword in each year to its highest frequency in a year. The B and D values represent the ratio of the total frequency of the keyword from 2000 to a certain year to its total frequency.
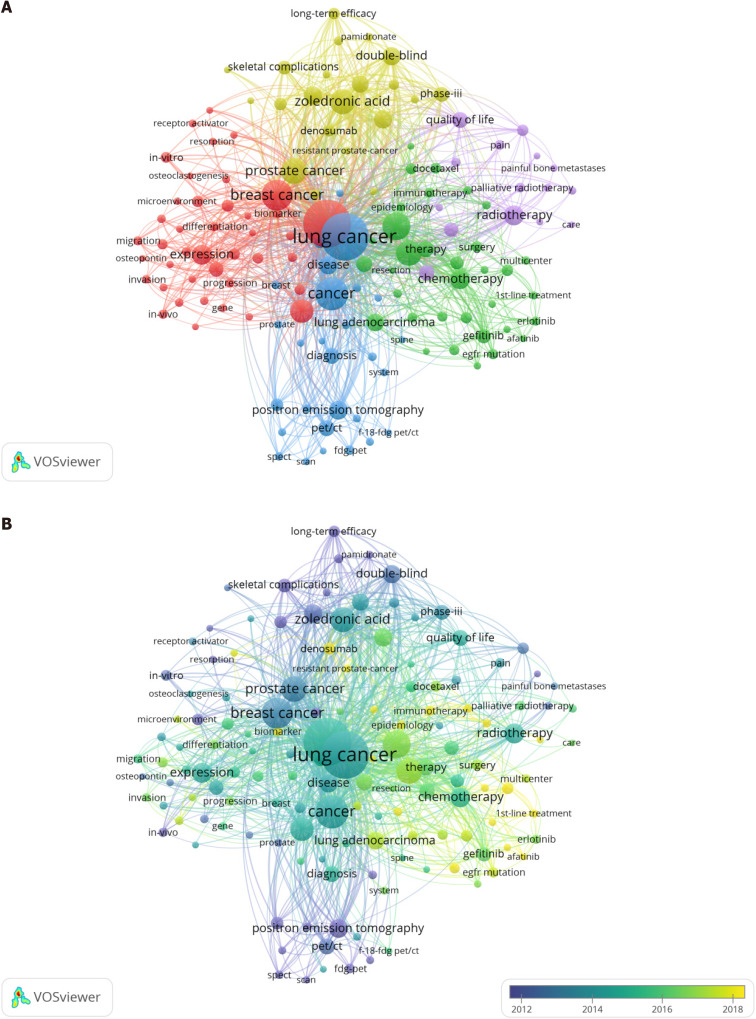
Keyword co-occurrence analysis of articles on LCBM: The construction of an LCBM keyword co-occurrence network is beneficial for an in-depth reproduction of the composition of LCBM knowledge. We extracted 139 high-frequency keywords with over 20 occurrences from 1903 articles for the co-occurrence network construction. The network can be divided into five categories (Figure 6): Cluster 1, basic research (red), including resorption, hormone-related protein, microenvironment and osteoclastogenesis; cluster 2, clinical treatment and related experiments (green), including gefitinib, immunotherapy and chemotherapy, where keywords on immunotherapy and targeted therapy are emerging; cluster 3, clinical diagnostic-related studies (blue), including diagnosis, positron emission tomography (PET) and fluorine-18-fluorodeoxyglucose PET/computed tomography (PET/CT); cluster 4, adjuvant therapy (yellow), including skeletal-related events, zoledronic acid and denosumab; and cluster 5, palliative care (purple), including painful BM and palliative radiotherapy. In addition, we found that some mechanisms, such as EGFR mutation, microRNA and tyrosine kinase inhibitors, were explored and distributed across the clusters.
Figure 6.
Analysis of the co-occurrence of all keywords. A: Network visualization map; B: Overlay visualization map. The small circle represents the keyword. The area of the small circle represents the frequency of the keyword. The colors of the different areas represent their categories. The lines of the connecting circles represent keywords that appear in an article at the same time.
Exploration of the molecular biological mechanisms of LCBM
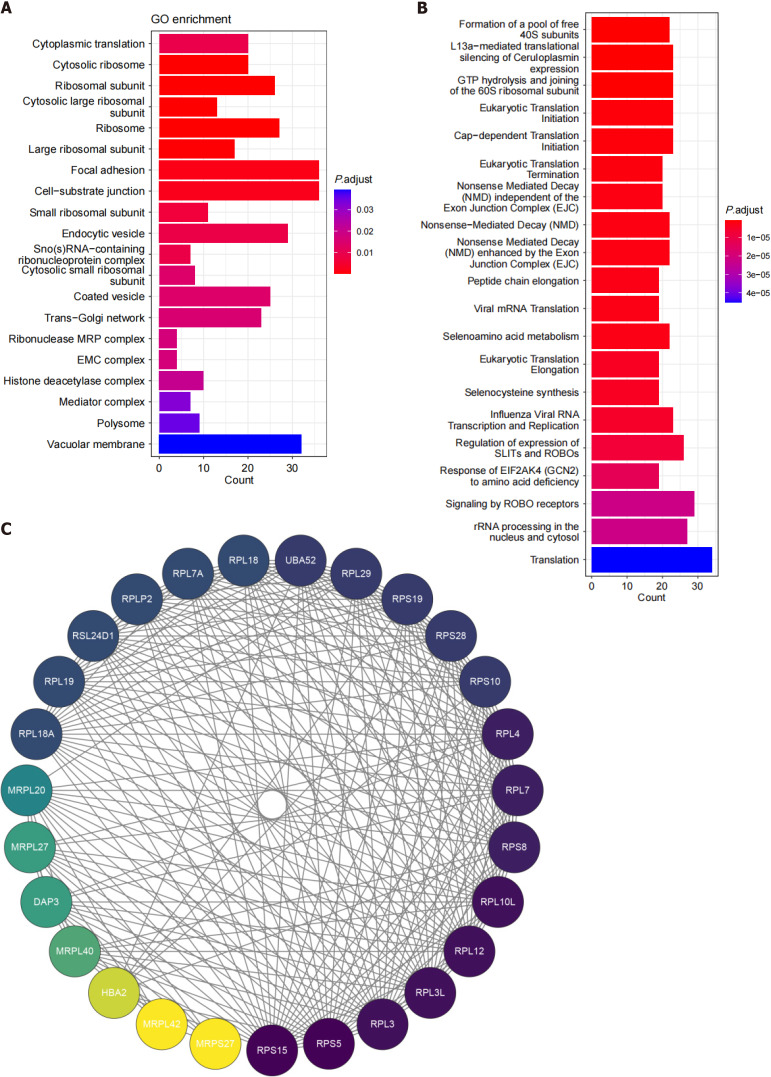
We screened 1238 DEGs from the dataset, including 606 upregulated genes and 632 downregulated genes. GO and Reactome functional annotations revealed that the functions of the DEGs in LCBM are mainly focused on the ribosome, the cytosolic ribosome, the formation of the pool of free 40S subunits related to ribosome-related pathways and focal adhesion related to cell adhesion migration (Figure 7). A PPI analysis of the ribosome-related DEGs in LCBM revealed that this pathway is mainly an interaction between the 60S ribosomal protein large subunit (RPL) and 40S ribosomal protein small subunit (RPS) families.
Figure 7.
Molecular map of lung cancer bone metastasis. A: Bubble map of differentially expressed genes (DEGs) based on the Gene Ontology enrichment analysis; B: Bubble map of DEGs based on the Reactome enrichment analysis; C: Protein-protein interaction network diagram related to the ribosome pathway. GO: Gene Ontology.
DISCUSSION
BM from primary tumours often suggest a poor prognosis[3,10]. Researchers have long been devoted to studying the basis and mechanisms of LCBM. With the development of high-throughput sequencing, this body of knowledge has exploded. This study is the first to organize and quantify the current large and confusing knowledge structure to provide suggestions for further LCBM research.
Changes in LCBM knowledge volume and country/region distribution
More systematic and precise treatments with immunotherapy and targeted therapy have helped to improve significantly the prognosis in LC, but BM remains a critical problem in these patients[11]. The worldwide LC patient population is very large[12]. In addition, approximately 350000 deaths are caused by BM in the United States each year[13]. This has objectively driven scholars to explore and develop the study of LCBM. The major contributors to LCBM research are primarily in developed countries, reflecting the level of social development needed to promote science and technology. However, some developing countries have emerged in recent years as forces that play an important role in this research. This is helpful for alleviating the dilemma facing developing countries of backward medical technology amid high morbidity and mortality rates for LCBM[12]. Furthermore, developed countries should strengthen their future cooperation efforts with developing countries to help resolve this dilemma (Supplementary Figure 2).
Changes in LCBM research hotspots
At the same time, the development of the knowledge on LCBM has been iterated owing to the continuous exploration and attention of researchers worldwide. Zoledronic acid, quality of life and skeletal-related events constitute the motor themes, suggesting that although neither denosumab nor zoledronic acid can change the outcomes and side effects of patients with LCBM, they can effectively reduce the skeletal-related events associated with a poor prognosis[14,15]. Palliative, palliative radiotherapy and chemotherapy constitute a falling theme that may be related to their relatively limited scope of action, high toxicity, myelosuppression and low efficacy[16,17]. On the other hand, the emergence of targeted therapies is related to the large amount of basic research on EGFR. A previous study claimed that EGFR is mutated in approximately 70% of patients with LCBM[18]. Specifically, in LCBM cells, epidermal growth factor or Wnt signalling pathways stimulate high ROR1 expression levels to interact with EGFR, which in turn promotes cancer cell proliferation, survival and invasion[19]. In addition, acquired resistance generated by first- and second-generation EGFR-tyrosine kinase inhibitors is closely associated with the behaviour of genes, such as the T790M mutation, MET amplification, AXL activation, IGF1R blockade, HER2 amplification, PTEN deletion and PIK3CA mutation[20]. These results invariably drive the creation of more effective EGFR-targeted therapies. The clinical application of immunotherapy is dependent on the maturation of research on immune mechanisms and the promotion of immune checkpoint inhibitors[21]. These are also examples of the basic research that drives clinical progress. Therefore, it is necessary to strengthen the basic research in the future to improve and clarify the relevant molecular mechanisms in LCBM. In recent years, the maturation of computer and other technologies, such as high-throughput sequencing, has enabled bioinformatics to become widely used in the study of LCBM. For example, in vitro models of LC cell-derived exocysts have been constructed to predict their BM capacity using methods such as meta-analysis[22]. Using meta-analyses, Sharma et al[23] and Chen et al[24] evaluated the efficacy of brachytherapy as compared with denosumab and zoledronic acid therapies in patients with LCBM. In an analysis of data from 8067 patients with advanced NSCLC, the use of programmed cell death protein 1 inhibitors in patients with BM was found to have no significant altering effect on progression-free survival[25]. In addition, the log-rank test, multiple Cox regression analysis and Kaplan-Meier method are other common research tools[26]. These studies are important for research on the construction of prognostic models, assessment of clinical outcomes and development of molecular diagnoses and treatments. This suggests that bioinformatic analysis may presently be an effective method for studying LCBM; however, most studies still lack further experimental validation.
In addition, as long-standing topics of interest in the field of LCBM, lung adenocarcinoma, non-small cell LC and so on reflect the large patient base of non-small cell LC, which is the most common type of LC[12,27]. However, brain and other metastases have also emerged as themes as they share some common problems. For example, both BM and brain metastasis in LC are closely related to the interaction of various components of the LC microenvironment, such as the epithelial-mesenchymal transition[28,29].
Main research directions in LCBM research
The volume of knowledge in LCBM research has been enriched gradually, mainly in the following areas. In terms of mechanism exploration, researchers have started to explore and identify key LCBM molecules through technological advancements, such as high-throughput sequencing. For example, Miki et al[30] and Liu et al[31] found that high expression levels of zinc finger E-box binding homology box 1 in LC cells leads to increased expression levels of parathyroid hormone-related protein, which prompts LC cells to undergo a continuous process of retention in the capillary bed, angiogenesis and proliferation, and causes the emergence of the site of metastasis, then metastasis to bone and an increase the number of BMs in the body. BM incidence is also increasing. In addition, primary tumours secrete several different factors and extracellular vesicles (EVs) into the circulation to reach bone, altering the bone microenvironment through mechanisms such as the action of calmodulin 11 in EVs with osteoblasts, eventually forming a pre-metastatic ecological niche in bone and preparing the tumour for BM[32,33]. When LC cells reach bone, since LC cells cannot directly damage the bone and colonize it, they must disrupt the RANKL/RANK/OPG pathway that forms a dynamic balance between osteogenesis and bone resorption, which is regulated by osteoblasts and osteoclasts. In this case, the action of the osteoclasts prevails and triggers the corresponding bone destruction and resorption, allowing LC cells to colonize and further invade and grow[34,35]. In addition, the mechanism of LCBM is similar to that of breast cancer BM[1]; thus, it is not surprising that the term “breast cancer” appears in our findings (Figure 6).
In terms of clinical treatments, the clinical exploration of targeted therapies and immunotherapies is in full swing. Numerous clinical studies have shown that both targeted therapy and immunotherapy can significantly extend survival and improve the prognosis of LCBM patients[36,37]. Among these, nivolumabtherapy may be effective in preventing bone destruction and relieving bone pain and other symptoms with long-term efficacy[38]. This is important for improving the quality of life for LCBM patients. Nevertheless, targeted therapies and immunotherapies for LCBM still face challenges, such as still being in the animal testing stage or drug resistance, and there is still a long way to go before they can be used as effective LCBM treatments[38-40].
Although palliative treatments have not been shown to make a significant positive impact on the survival of patients with BM, they can be highly effective for improving patient quality of life by providing rapid symptom relief[41,42]. Therefore, considering the unsatisfactory results of emerging therapies and the current absence of viable cures, palliative care is considered the best option for patients with BM to maintain a relatively high quality of life at the end of life[43].
Moreover, although adjuvant treatments, such as denosumab and zoledronic acid, can induce serious side effects, including hypocalcaemia, femoral fractures and osteonecrosis of the jaw, a large body of data suggests that they can play a significant role in prolonging the overall survival of LCBM patients and reducing the occurrence of a range of skeletal complications[14,15,44]. Therefore, it is not surprising that these treatments are major hot topics in LCBM research.
For clinical diagnosis, the commonly used PET/CT is useful for providing an accurate assessment of the risk LC patients have for developing BM and has great potential for therapeutic guidance[45,46]. This suggests that PET/CT may continue as a hot topic in the future. In addition, the scattering of certain mechanisms in the distribution of the abovementioned topics and the extensive connections with many application-related topics may reflect the fact that the relevant basic results are already driving the development of clinical applications.
Preliminary exploration of the molecular mechanisms of LCBM
The updated global molecular map of LCBM that we constructed suggests that the factors affecting the development of BM in LC patients may be closely related to abnormalities in the ribosomal pathway. This is largely consistent with existing reports. It has been reported that RPLP2, which encodes the eukaryotic 60S large ribosomal subunit P2, is significantly upregulated in patients with LCBM and is an important marker of a poor prognosis in these patients[47]. RPLP2 is also associated with DNA repair, cell division, apoptosis and the progression of multiple types of tumorigenesis[47]. Not coincidentally, Ebright et al[48] found that mice with breast cancer and high RPL15 expression levels have a greater likelihood of breast cancer cell metastasis to multiple organs. In LCBM tissue, the upregulation of COL6A1, which is closely related to the ribosome-related pathways, can enhance the proliferation and invasion of tumour cells and their adhesion to osteoblasts[49]. The upregulation of SMYD2, a transcriptional regulator, can activate the transcription of ribosomal small subunit protein 7 by binding to its promoter, thereby promoting the proliferation, migration, invasion and distant metastasis of LC cells[50]. Small nucleolar RNA can enhance downstream SCD1 translation and regulate lipid peroxidation and mechanistic target of rapamycin by promoting the 2’-O-methylation modification of the C3680 site on 28S rRNA to inhibit autophagy of LC cells and thereby promote LC cell growth and metastasis[51]. Ribosomal S6 kinase 2 can jointly promote CaMKIV signal transduction and enhance CREB activity through interaction with GDH1, thereby promoting LC cell metastasis[52]. More importantly, there are also in vivo experiments showing that the use of biomineralized metal-organic framework nanoparticles with protein toxins and nuclear factor-κB ligand receptor activator antibodies can reduce the occurrence of BM through the irreversible inactivation of ribosomes, a decrease in the number of LC cells and an interference with the interaction between LC cells and bone cells[53]. These indicate that ribosomes are very likely to be an important link in the occurrence of BM by LC cells and the interaction between LC cells and bone cells to cause skeletal-related events. This also reveals the clinical potential of this dual-protein therapy in the treatment of LC and LCBM. Furthermore, Bowley et al[54] found that high expression levels of RPL and RPS lead to aberrant ribosome synthesis that can promote the onset and progression of melanoma brain metastasis. A 20-year empirical report indicated that Diamond-Blackfan anaemia patients, which is caused by RPL or RPS mutations, has a high susceptibility to malignancy[55]. This is similar to the interaction between the RPS and RPL families in the results of the PPI analysis of the ribosome-related pathways. However, no direct evidence has confirmed that the ribosomal pathway in LC cells can also trigger BM through a similar action. In addition, focal adhesion-related pathways may also be closely associated with the BM of LC cells. One study that used microarray fractionation identified long-stranded noncoding RNAs that may mediate the development of spinal metastasisby affecting processes such as focal and cell adhesions in lung adenocarcinoma cells[56]. Another study claimed that the focal adhesion kinase/AKT/Rho-associated kinase pathway induces LC cell invasion and metastasis by interacting with bone bridge proteins, thus effectively inhibiting some protein activity[57]. These findings are similar to those of our enrichment analysis and fully suggest that focal adhesion may play a BM-promoting role in LC.
In addition, these pathways also play a role in other tumours or metastases. For example, the interaction between focal adhesion and extracellular matrix receptors can lead to changes in the actin cytoskeletal pathway and thereby promote the migration of LC cells to the brain[58]. MiR-215-5p downregulates the metastatic ability of colorectal cancer cells by regulating molecular biological processes, such as extracellular matrix-receptor interactions and focal adhesion[59]. The study revealed that miR-215-5p is likely to become a potential target for the treatment of the distant metastases of colorectal cancer. FSTL1 interacts with VIM to activate the focal adhesion signalling pathway and cytoskeleton rearrangement to achieve colorectal cancer liver metastasis[60]. EIF4A2, which is required for the binding of mRNA to ribosomes, plays an important role in the lung metastasis of colorectal cancer cells in vivo[61]. The significant molecular biological characteristics of LC brain metastasis include the significant enhancement of pathways related to ribosome activity and metabolic reprogramming[62]. In addition, there are studies claiming that ribosome biogenesis is a target with a strong clinical potential for treating many tumour metastases and alleviating drug resistance[63]. This also further illustrates the rationality of ribosome and focal adhesion-related pathways playing a role in LCBM. However, whether similar mechanisms are achieved in LCBM and what specific biological processes involve these mechanisms in the expression of molecules, transcriptional regulation, epigenetic modification and their clinical potential are still unknown. In the future, we plan to conduct more research that further explores and improves the data on the related biological mechanisms.
However, not all of the abovementioned molecular biological mechanisms appeared directly in the results of the bibliometric analysis of this study. This may be due to the fact that much of the literature was included in this study for the bibliometric analysis but only one dataset was used in constructing the LCBM-related molecular map. However, we believe that the direction of the basic research on LCBM is still weak. For example, the few existing studies mentioned earlier have shown that ribosomal and focal adhesion-related pathways may be the link that promotes BM by LC cells. This link is promising as a new target for the treatment of LCBM. However, as of now, no mature studies have explained the detailed processes of these pathways and developed clinical therapeutic diagnostics based on them. This is also illustrated by the fact that a dataset met our filtering requirements in multiple worldwide datasets. In addition, we investigated GTP hydrolysis and the joining of the 60S ribosomal subunit, translation, viral mRNA translation and NMD-related pathways in LCBM. However, no study has directly confirmed this. This requires further experimental validation. Thus, while clinical studies and new drugs have helped improve the survival prognosis for LC, there remains an urgent need for larger population data to enrich the molecular landscape of LCBM and more representative animal models to explain the biological behaviour of LCBM. This will lay the foundation for further advancements in the development of more effective diagnostic and therapeutic options for LCBM.
Limitations of the study and future research arrangements
This study has the following shortcomings: (1) It included only articles written in English that were of high quality and whose data could be comprehensively examined from the WOSCC. In the future, we should also include an analysis of articles on LCBM from other databases for supplementation and validation; (2) The study addressed only the current mainstream evolution of LCBM, hot directions, the current status of research on LCBM and future directions using limited analysis. We will further examine the valuable information behind these bibliometric indicators in the future; and (3) Although this study used various methods to analyse only the data sets that met the inclusion requirements for investigating the possible biological behaviour of LCBM and obtained strong suggestive and pioneering information, it lacks the verification of in vivo and in vitro experiments. In the future, we should conduct in vivo and ex vivo experiments to confirm the molecular biological mechanisms in LCBM.
CONCLUSION
In summary, LCBM has received extensive attention in recent years owing to targeted therapies and immunotherapies on the clinical research side and drug resistance mechanisms on the basic research side. Although the current regimens can provide improvements in patient quality of life, they cannot change the outcomes in patients with LCBM. This is mainly related to the poor effects of novel therapies and weak basic research. Future scientific research on LCBM can increase efforts on targeted therapies, immunotherapies and basic research to provide new concepts for the study of LCBM mechanisms and improve its prognosis.
ACKNOWLEDGEMENTS
Thanks to all the software and “Guangxi Key Laboratory of Medical Pathology” for providing technical support in computational pathology.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Watanabe T, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhao YQ
Contributor Information
Yi Chen, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Xiao-Song Chen, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Rong-Quan He, Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Zhi-Guang Huang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Hui-Ping Lu, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Hong Huang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Da-Ping Yang, Department of Pathology, Guigang People’s Hospital of Guangxi/The Eighth Affiliated Hospital of Guangxi Medical University, Guigang 537100, Guangxi Zhuang Autonomous Region, China.
Zhong-Qing Tang, Department of Pathology, Wuzhou Gongren Hospital/The Seventh Affiliated Hospital of Guangxi Medical University, Wuzhou 543000, Guangxi Zhuang Autonomous Region, China.
Xia Yang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Han-Jie Zhang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Ning Qv, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Jin-Liang Kong, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Gang Chen, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China. chen_gang_triones@163.com.
References
- 1.Ben-Ghedalia-Peled N, Vago R. Wnt Signaling in the Development of Bone Metastasis. Cells. 2022;11 doi: 10.3390/cells11233934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouveia AG, Chan DCW, Hoskin PJ, Marta GN, Trippa F, Maranzano E, Chow E, Silva MF. Advances in radiotherapy in bone metastases in the context of new target therapies and ablative alternatives: A critical review. Radiother Oncol. 2021;163:55–67. doi: 10.1016/j.radonc.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Knapp BJ, Devarakonda S, Govindan R. Bone metastases in non-small cell lung cancer: a narrative review. J Thorac Dis. 2022;14:1696–1712. doi: 10.21037/jtd-21-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Yang W, Liu Q. Cancer challenges worldwide and in China: preparing for the inevitable. Sci China Life Sci. 2022;65:442–444. doi: 10.1007/s11427-021-2009-0. [DOI] [PubMed] [Google Scholar]
- 5.Cavers D, Nelson M, Rostron J, Robb KA, Brown LR, Campbell C, Akram AR, Dickie G, Mackean M, van Beek EJR, Sullivan F, Steele RJ, Neilson AR, Weller D. Understanding patient barriers and facilitators to uptake of lung screening using low dose computed tomography: a mixed methods scoping review of the current literature. Respir Res. 2022;23:374. doi: 10.1186/s12931-022-02255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Guo Z, Zou Z, Alswadeh M, Wang H, Liu X, Li X. Development and validation of a prognostic nomogram for bone metastasis from lung cancer: A large population-based study. Front Oncol. 2022;12:1005668. doi: 10.3389/fonc.2022.1005668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Zhu XP, Shi JJ, Yuan GZ, Yao ZA, Chu YG, Shi S, Jia QL, Chen T, Hu YH. Coronary Heart Disease and Depression or Anxiety: A Bibliometric Analysis. Front Psychol. 2021;12:669000. doi: 10.3389/fpsyg.2021.669000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Cheng K, Guo Q, Yang W, Tong L, Wang Y, Sun Z. Mapping Knowledge Structure and Themes Trends of Osteoporosis in Rheumatoid Arthritis: A Bibliometric Analysis. Front Med (Lausanne) 2021;8:787228. doi: 10.3389/fmed.2021.787228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokol P, Blažun Vošner H, Završnik J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Info Libr J. 2021;38:125–138. doi: 10.1111/hir.12295. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y, Lv Y. Dual targeted zeolitic imidazolate framework nanoparticles for treating metastatic breast cancer and inhibiting bone destruction. Colloids Surf B Biointerfaces. 2022;219:112826. doi: 10.1016/j.colsurfb.2022.112826. [DOI] [PubMed] [Google Scholar]
- 11.Zulfiqar B, Farooq A, Kanwal S, Asghar K. Immunotherapy and targeted therapy for lung cancer: Current status and future perspectives. Front Pharmacol. 2022;13:1035171. doi: 10.3389/fphar.2022.1035171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 13.Huang JF, Shen J, Li X, Rengan R, Silvestris N, Wang M, Derosa L, Zheng X, Belli A, Zhang XL, Li YM, Wu A. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann Transl Med. 2020;8:482. doi: 10.21037/atm.2020.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozzo A, Deng J, Abbas U, Bhasin R, Deodat M, Wariach S, Sanger S, Axelrod D, Masrouha K, Turcotte R, Wilson D, Ghert M. Which Bone-Modifying Agent is Associated with Better Outcomes in Patients with Skeletal Metastases from Lung Cancer? A Systematic Review and Network Meta-analysis. Clin Orthop Relat Res. 2021;479:2047–2057. doi: 10.1097/CORR.0000000000001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Wei J, Ge Q, Xing D, Zhou X, Qian Y, Jiang G. The optimized drug delivery systems of treating cancer bone metastatic osteolysis with nanomaterials. Drug Deliv. 2021;28:37–53. doi: 10.1080/10717544.2020.1856225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieder C. Second re-irradiation: A delicate balance between safety and efficacy. Phys Med. 2019;58:155–158. doi: 10.1016/j.ejmp.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Pan Y, Mao Y, Chen Y, He Y. Current progress and mechanisms of bone metastasis in lung cancer: a narrative review. Transl Lung Cancer Res. 2021;10:439–451. doi: 10.21037/tlcr-20-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng A, Li Y, Li G, Wang Y, Wen Q, Yang Z, Tian K, Lv H, Guo L, Zhang S, Liu X, Jiang D. Genomic Features of Organ-Specific Metastases in Lung Adenocarcinoma. Front Oncol. 2022;12:908759. doi: 10.3389/fonc.2022.908759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Wang S, Xing Z, Lin A, Liang K, Song J, Hu Q, Yao J, Chen Z, Park PK, Hawke DH, Zhou J, Zhou Y, Zhang S, Liang H, Hung MC, Gallick GE, Han L, Lin C, Yang L. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106–119. doi: 10.1038/ncb3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1–10. doi: 10.1016/j.ctrv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Wang M, Xu C, Li B, Chen J, Wang Z. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J Immunol Res. 2021;2021:8970173. doi: 10.1155/2021/8970173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhadresha K, Upadhyay V, Brahmbhatt J, Mughal MJ, Jain N, Rawal R. In vitro model of predicting metastatic ability using tumor derived extracellular vesicles; beyond seed soil hypothesis. Sci Rep. 2022;12:20258. doi: 10.1038/s41598-022-24443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma R, Sagoo NS, Haider AS, Sharma N, Haider M, Sharma IK, Igbinigie M, Aya KL, Aoun SG, Vira S. Iodine-125 radioactive seed brachytherapy as a treatment for spine and bone metastases: A systematic review and meta-analysis. Surg Oncol. 2021;38:101618. doi: 10.1016/j.suronc.2021.101618. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Zhou L, Liu X, Wen X, Li H, Li W. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int J Clin Pharm. 2021;43:2–10. doi: 10.1007/s11096-020-01105-1. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Zhu L, Guo T, Chen W, Zhang Z, Li W, Pan X. Metastatic sites as predictors in advanced NSCLC treated with PD-1 inhibitors: a systematic review and meta-analysis. Hum Vaccin Immunother. 2021;17:1278–1287. doi: 10.1080/21645515.2020.1823779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X, Ma W, Wu H, Xu Y, Wang D, Zhang S, Liu Z, Chekhonin VP, Peltzer K, Zhang J, Wang X, Zhang C. Synchronous bone metastasis in lung cancer: retrospective study of a single center of 15,716 patients from Tianjin, China. BMC Cancer. 2021;21:613. doi: 10.1186/s12885-021-08379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Huang M, Xie W, Ding Q, Wang T. Eupafolin regulates non-small-cell lung cancer cell proliferation,migration, and invasion by suppressing MMP9 and RhoA via FAK/PI3K/AKT signaling pathway. J Biosci. 2023;48 [PubMed] [Google Scholar]
- 28.Han L, Huang Z, Liu Y, Ye L, Li D, Yao Z, Wang C, Zhang Y, Yang H, Tan Z, Tang J, Yang Z. MicroRNA-106a regulates autophagy-related cell death and EMT by targeting TP53INP1 in lung cancer with bone metastasis. Cell Death Dis. 2021;12:1037. doi: 10.1038/s41419-021-04324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015;121:101–108. doi: 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Yano S, Hanibuchi M, Kanematsu T, Muguruma H, Sone S. Parathyroid hormone-related protein (PTHrP) is responsible for production of bone metastasis, but not visceral metastasis, by human small cell lung cancer SBC-5 cells in natural killer cell-depleted SCID mice. Int J Cancer. 2004;108:511–515. doi: 10.1002/ijc.11586. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Zhang N, Wang Y, Xu M, Liu N, Pang X, Cao J, Ma N, Pang H, Liu L, Zhang H. Zinc finger E-box binding homeobox 1 promotes invasion and bone metastasis of small cell lung cancer in vitro and in vivo. Cancer Sci. 2012;103:1420–1428. doi: 10.1111/j.1349-7006.2012.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He G, Nie JJ, Liu X, Ding Z, Luo P, Liu Y, Zhang BW, Wang R, Hai Y, Chen DF. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact Mater. 2023;19:690–702. doi: 10.1016/j.bioactmat.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Q, Wang D, Feng J, Guo P, Geng C. Denosumab inhibits MCF-7 cell line-induced spontaneous osteoclastogenesis via the RANKL/MALAT1/miR-124 axis. Transl Cancer Res. 2020;9:2482–2491. doi: 10.21037/tcr.2020.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen F, Han Y, Kang Y. Bone marrow niches in the regulation of bone metastasis. Br J Cancer. 2021;124:1912–1920. doi: 10.1038/s41416-021-01329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gen S, Tanaka I, Morise M, Koyama J, Kodama Y, Matsui A, Miyazawa A, Hase T, Hibino Y, Yokoyama T, Kimura T, Yoshida N, Sato M, Hashimoto N. Clinical efficacy of osimertinib in EGFR-mutant non-small cell lung cancer with distant metastasis. BMC Cancer. 2022;22:654. doi: 10.1186/s12885-022-09741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng C, Ge X, Tian J, Wei J, Zhao L. Prognostic role of targeted therapy in patients with multiple-site metastases from non-small- cell lung cancer. Future Oncol. 2020;16:1957–1967. doi: 10.2217/fon-2020-0289. [DOI] [PubMed] [Google Scholar]
- 38.Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, Tao X, Mirando AJ, Hilton MJ, Ji RR. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. 2020;130:3603–3620. doi: 10.1172/JCI133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosoya K, Fujimoto D, Morimoto T, Kumagai T, Tamiya A, Taniguchi Y, Yokoyama T, Ishida T, Matsumoto H, Hirano K, Kominami R, Tomii K, Suzuki H, Hirashima T, Tanaka S, Uchida J, Morita M, Kanazu M, Mori M, Nagata K, Fukuda I, Tamiya M. Clinical factors associated with shorter durable response, and patterns of acquired resistance to first-line pembrolizumab monotherapy in PD-L1-positive non-small-cell lung cancer patients: a retrospective multicenter study. BMC Cancer. 2021;21:346. doi: 10.1186/s12885-021-08048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamiya M, Fujikawa K, Suzuki H, Yokoyama T, Uenami T, Tamiya A, Sato Y, Saito G, Uchida J, Morita M, Hirashima T, Fukuda Y, Kanazu M, Hosoya K, Suzuki T, Ueno K, Fujimoto D, Kumagai T, Teramukai S. Classification and regression tree for estimating predictive markers to detect T790M mutations after acquired resistance to first line EGFR-TKI: HOPE-002. Invest New Drugs. 2022;40:361–369. doi: 10.1007/s10637-021-01203-5. [DOI] [PubMed] [Google Scholar]
- 41.Leto G. Current status and future directions in the treatment of bone metastases from breast cancer. Clin Exp Pharmacol Physiol. 2019;46:968–971. doi: 10.1111/1440-1681.13139. [DOI] [PubMed] [Google Scholar]
- 42.Napoli A, De Maio A, Alfieri G, Gasperini C, Scipione R, Campanacci L, Siepe G, De Felice F, Siniscalchi B, Chiurchioni L, Tombolini V, Donati DM, Morganti AG, Ghanouni P, Catalano C, Bazzocchi A. Focused Ultrasound and External Beam Radiation Therapy for Painful Bone Metastases: A Phase II Clinical Trial. Radiology. 2023;307:e211857. doi: 10.1148/radiol.211857. [DOI] [PubMed] [Google Scholar]
- 43.Ning MS, Das P, Rosenthal DI, Dabaja BS, Liao Z, Chang JY, Gomez DR, Klopp AH, Gunn GB, Allen PK, Nitsch PL, Natter RB, Briere TM, Herman JM, Wells R, Koong AC, McAleer MF. Early and Midtreatment Mortality in Palliative Radiotherapy: Emphasizing Patient Selection in High-Quality End-of-Life Care. J Natl Compr Canc Netw. 2021;19:805–813. doi: 10.6004/jnccn.2020.7664. [DOI] [PubMed] [Google Scholar]
- 44.Cheung E, Borno HT. The limitations of today's clinical guidance: Atypical femoral fracture and long-term bone-modifying agents in the oncology setting. J Oncol Pharm Pract. 2020;26:1180–1189. doi: 10.1177/1078155220907965. [DOI] [PubMed] [Google Scholar]
- 45.Lim CH, Ahn TR, Moon SH, Cho YS, Choi JY, Kim BT, Lee KH. PET/CT features discriminate risk of metastasis among single-bone FDG lesions detected in newly diagnosed non-small-cell lung cancer patients. Eur Radiol. 2019;29:1903–1911. doi: 10.1007/s00330-018-5764-9. [DOI] [PubMed] [Google Scholar]
- 46.Mankoff DA, Katz SI. PET imaging for assessing tumor response to therapy. J Surg Oncol. 2018;118:362–373. doi: 10.1002/jso.25114. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Sun Y, Sun J, Wang Z, Zhou Y, Yao G, Gu Y, Zhang H, Zhao H. Differentially expressed and survival-related proteins of lung adenocarcinoma with bone metastasis. Cancer Med. 2018;7:1081–1092. doi: 10.1002/cam4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebright RY, Lee S, Wittner BS, Niederhoffer KL, Nicholson BT, Bardia A, Truesdell S, Wiley DF, Wesley B, Li S, Mai A, Aceto N, Vincent-Jordan N, Szabolcs A, Chirn B, Kreuzer J, Comaills V, Kalinich M, Haas W, Ting DT, Toner M, Vasudevan S, Haber DA, Maheswaran S, Micalizzi DS. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science. 2020;367:1468–1473. doi: 10.1126/science.aay0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Liu M, Cao X, Li W, Li Y, Zhao Z. Identification of differentially expressed genes using microarray analysis and COL6A1 induction of bone metastasis in non-small cell lung cancer. Oncol Lett. 2021;22:693. doi: 10.3892/ol.2021.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L, Kou F, Ji Z, Li B, Zhang B, Guo Y, Yang L. SMYD2 promotes tumorigenesis and metastasis of lung adenocarcinoma through RPS7. Cell Death Dis. 2021;12:439. doi: 10.1038/s41419-021-03720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K, Wang S, Zhang Y, Xie L, Song X. SNORD88C guided 2'-O-methylation of 28S rRNA regulates SCD1 translation to inhibit autophagy and promote growth and metastasis in non-small cell lung cancer. Cell Death Differ. 2023;30:341–355. doi: 10.1038/s41418-022-01087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang J, Chun J, Hwang JS, Pan C, Li J, Boese AC, Young I, Malin CM, Kang Y, Gibbons DL, Sica G, Fu H, Ramalingam SS, Jin L, Kang S. EGFR-phosphorylated GDH1 harmonizes with RSK2 to drive CREB activation and tumor metastasis in EGFR-activated lung cancer. Cell Rep. 2022;41:111827. doi: 10.1016/j.celrep.2022.111827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niu Y, Yang H, Yu Z, Gao C, Ji S, Yan J, Han L, Huo Q, Xu M, Liu Y. Intervention with the Bone-Associated Tumor Vicious Cycle through Dual-Protein Therapeutics for Treatment of Skeletal-Related Events and Bone Metastases. ACS Nano. 2022;16:2209–2223. doi: 10.1021/acsnano.1c08269. [DOI] [PubMed] [Google Scholar]
- 54.Bowley TY, Lagutina IV, Francis C, Sivakumar S, Selwyn RG, Taylor E, Guo Y, Fahy BN, Tawfik B, Marchetti D. The RPL/RPS gene signature of melanoma CTCs associates with brain metastasis. Cancer Res Commun. 2022;2:1436–1448. doi: 10.1158/2767-9764.CRC-22-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quarello P, Garelli E, Carando A, Cillario R, Brusco A, Giorgio E, Ferrante D, Corti P, Zecca M, Luciani M, Pierri F, Putti MC, Cantarini ME, Farruggia P, Barone A, Cesaro S, Russo G, Fagioli F, Dianzani I, Ramenghi U AIEOP working group on Diamond Blackfan Anaemia. A 20-year long term experience of the Italian Diamond-Blackfan Anaemia Registry: RPS and RPL genes, different faces of the same disease? Br J Haematol. 2020;190:93–104. doi: 10.1111/bjh.16508. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Hu A, Liang Y, Wang K, Zhou X, Dong J. Genome-wide analysis of long non-coding RNA expression profile in lung adenocarcinoma compared to spinal metastasis. Ann Transl Med. 2020;8:1516. doi: 10.21037/atm-20-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang CG, Im E, Lee HJ, Lee EO. Plumbagin reduces osteopontin-induced invasion through inhibiting the Rho-associated kinase signaling pathway in A549 cells and suppresses osteopontin-induced lung metastasis in BalB/c mice. Bioorg Med Chem Lett. 2017;27:1914–1918. doi: 10.1016/j.bmcl.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 58.Gao Y, Li G, Sun L, He Y, Li X, Sun Z, Wang J, Jiang Y, Shi J. ACTN4 and the pathways associated with cell motility and adhesion contribute to the process of lung cancer metastasis to the brain. BMC Cancer. 2015;15:277. doi: 10.1186/s12885-015-1295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machackova T, Vychytilova-Faltejskova P, Souckova K, Trachtova K, Brchnelova D, Svoboda M, Kiss I, Prochazka V, Kala Z, Slaby O. MiR-215-5p Reduces Liver Metastasis in an Experimental Model of Colorectal Cancer through Regulation of ECM-Receptor Interactions and Focal Adhesion. Cancers (Basel) 2020;12 doi: 10.3390/cancers12123518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu C, Wang X, Long T, Zhong Y, Ma Y, Hu Z, Li Z. FSTL1 interacts with VIM and promotes colorectal cancer metastasis via activating the focal adhesion signalling pathway. Cell Death Dis. 2018;9:654. doi: 10.1038/s41419-018-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen ZH, Qi JJ, Wu QN, Lu JH, Liu ZX, Wang Y, Hu PS, Li T, Lin JF, Wu XY, Miao L, Zeng ZL, Xie D, Ju HQ, Xu RH, Wang F. Eukaryotic initiation factor 4A2 promotes experimental metastasis and oxaliplatin resistance in colorectal cancer. J Exp Clin Cancer Res. 2019;38:196. doi: 10.1186/s13046-019-1178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woldmar N, Schwendenwein A, Kuras M, Szeitz B, Boettiger K, Tisza A, László V, Reiniger L, Bagó AG, Szállási Z, Moldvay J, Szász AM, Malm J, Horvatovich P, Pizzatti L, Domont GB, Rényi-Vámos F, Hoetzenecker K, Hoda MA, Marko-Varga G, Schelch K, Megyesfalvi Z, Rezeli M, Döme B. Proteomic analysis of brain metastatic lung adenocarcinoma reveals intertumoral heterogeneity and specific alterations associated with the timing of brain metastases. ESMO Open. 2023;8:100741. doi: 10.1016/j.esmoop.2022.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elhamamsy AR, Metge BJ, Alsheikh HA, Shevde LA, Samant RS. Ribosome Biogenesis: A Central Player in Cancer Metastasis and Therapeutic Resistance. Cancer Res. 2022;82:2344–2353. doi: 10.1158/0008-5472.CAN-21-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]