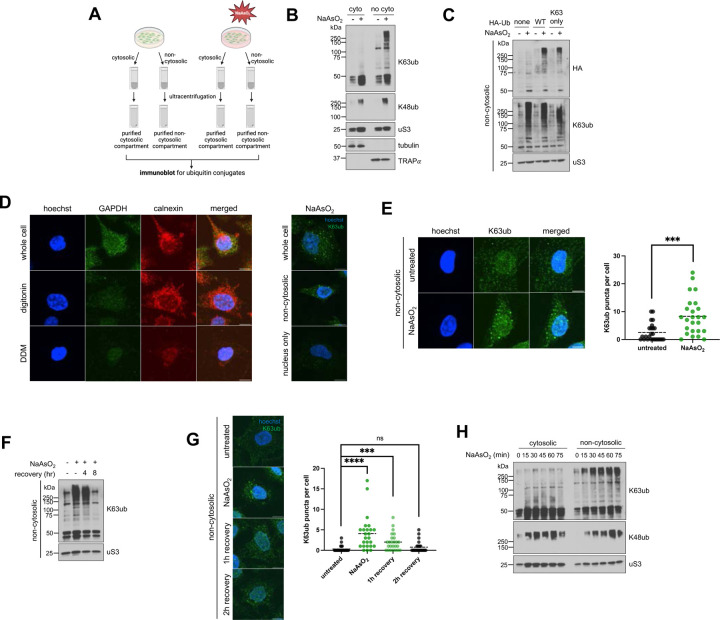
Figure 1. Arsenite stress promotes reversible non-cytosolic K63 ubiquitin accumulation.
1A – Schematic of the cytosolic and non-cytosolic fractionation and sucrose sedimentation approach. Cells were treated as described, then detergents were used to extract cytosolic fractions, followed by non-cytosolic fractions from cells attached to plate. Then fractions were then further purified by ultracentrifugation over a sucrose cushion.
1B – Arsenite stress induces local accumulation specifically for K63 ubiquitin signals in purified non-cytosoilc compartments. Immunoblots of anti-K63ub and anti-K48ub from fractions of cells treated with 1 hour of 0.5 mM NaAsO2. Anti-uS3 was used as a loading control for each fraction. Before ultracentrifugation, anti-tubulin and anti-TRAPα were used as fractionation controls for the cytosol and non-cytosolic fractions, respectively.
1C – Exogenous HA-Ub(K63) accumulates in the non-cytosolic fraction upon arsenite stress. Immunoblot of anti-HA and anti-K63ub from non-cytosolic fractions of cells transfected with either WT or K63-only HA-ubiquitin plasmids, then treated with 1 hour of 0.5 mM NaAsO2. Anti-uS3 was used as a loading control.
1D – Approach for observing non-cytosolic K63ub puncta dynamics. Immunofluorescence microscopy of anti-GAPDH (cytosol) and anti-calnexin (ER/membrane) from HeLa cells prepared either as whole cells, cytosol-depleted cells after a digitonin wash, or membrane-depleted cells after a digitonin and DDM wash. Hoechst was used to identify and count cells. Protocol was repeated on the right after treatment of 1 hour 0.5mM NaAsO2, then stained for anti-K63ub. Hoechst was used to identify cells. Scale bar = 10 μm.
1E – Non-cytosolic K63ub linkages form puncta upon arsenite stress. Immunofluorescence microscopy of anti-K63ub from cells treated with 1 hour of 0.5 mM NaAsO2. Cells were prepared with a digitonin wash to release cytosolic components before fixation. Hoechst was used to identify and count cells. Analysis included 25 cells per group. Scale bar = 10 μm.
1F – Non-cytosolic K63ub accumulation is reversible after arsenite washout. Immunoblot of anti-K63ub from non-cytosolic fractions of cells along a course of treatment with 1 hour of 0.5 mM NaAsO2 with a washout and recovery of either 4 or 8 hours. Anti-uS3 was used as a loading control.
1G – Non-cytosolic K63ub puncta formation is reversible after arsenite washout. Immunofluorescence microscopy of anti-K63ub from cells along a course of treatment with 1 hour of 0.5 mM NaAsO2 with a washout and recovery of either 1, 2, or 4 hours. Cells were prepared with a digitonin wash to release cytosolic components before fixation. Hoechst was used to identify and count cells. Analysis included 25 cells per group. Scale bar = 10 μm.
1H – Arsenite stress does not induce accumulation of K63 ubiquitin signals in purified cytosolic fractions. Immunoblots of anti-K63ub and anti-K48ub from fractions of cells treated along a 15-minute time course of 0.5 mM NaAsO2 up to 75 minutes. Anti-uS3 was used as a loading control for each purified fraction. Before ultracentrifugation, anti-tubulin and anti-TRAPα were used as fractionation controls for the cytosol and non-cytosolic fractions, respectively.