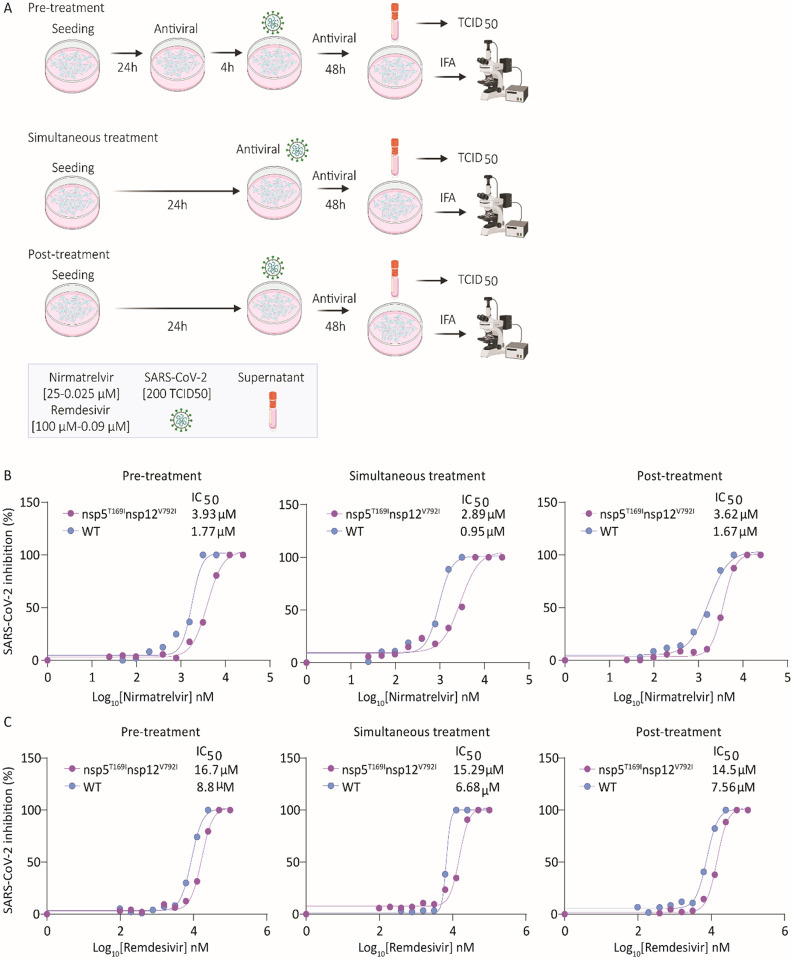
Fig. 2: Sensitivity of SARS-CoV-2-nsp5T169Insp12V792I virus to nirmatrelvir and remdesivir in vitro.
(A) Experimental layout showing the treatment plans to study the sensitivity of the SARS-CoV-2-nsp5T169Insp12V792I virus to antiviral therapies. (B) Nirmatrelvir resistance of SARS-CoV-2. Vero E6 cells were treated with increasing doses of nirmatrelvir (0.05-25 μM) following three treatment conditions (pre-, simultaneous, and post-treatment) and infected with 200 TCID50/well of SARS-CoV-2-nsp5T169Insp12V792I and WT viruses. Virus titers in supernatant of treated/infected cells was quantified by limiting dilution and the percent inhibition value was calculated to obtain the 50% inhibitory concentration (IC50). Results represent the average of 6 replicates and three independent experiments. (B) Remdesivir resistance of SARS-CoV-2. Vero E6 cells were treated with increasing doses (0.09-100 μM) of remdesivir following three treatment regimens and infected with 200 TCID50/well of SARS-CoV-2-nsp5T169Insp12V792I and WT isolates. Virus titers in supernatants was quantified by limiting dilution and the percent inhibition value was calculated to obtain the 50% inhibitory concentration (IC50). Results represent the average of four replicates and two independent experiments.