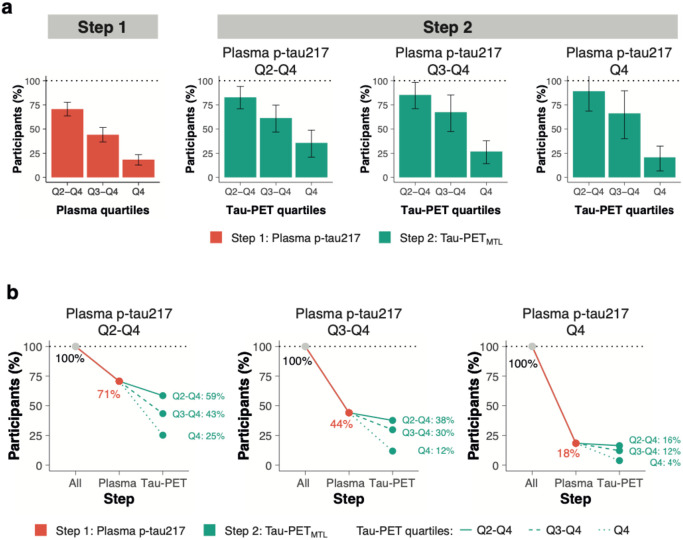
Figure 4. Clinical trial sample size reductions through a two-step recruitment strategy when using clinical progression to mild cognitive impairment as an outcome measure.
a, the obtained sample size reduction using sample selection based on different percentiles (75th, 50th and 25th) of baseline plasma p-tau217 levels in step 1 followed by the selection based on the same percentiles (75th, 50th and 25th) of the Tau-PETMTL measurement in step 2 with clinical progression to mild cognitive impairment as the primary endpoint. Note that 100% in step 2 refers to the participants selected by plasma p-tau217 in step 1. Errorbars represent the 95% CI. b shows the calculated sample size reductions for various plasma p-tau217 and Tau-PETMTL quartile combinations.