Graphical abstract
Keywords: Arrhythmogenic right ventricular cardiomyopathy, Mushroom-shaped right ventricular outflow tract aneurysm, Cardiomyopathy, Echocardiography
Highlights
-
•
ARVC is a life-threatening disease that requires early detection to prevent SCD.
-
•
ms-RVOT may serve as an early clue for diagnosis of ARVC.
-
•
ms-RVOT may help preempt adverse outcomes in asymptomatic ARVC cases.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited disease characterized by right ventricular (RV) dysfunction, ventricular arrhythmias, and risk of sudden cardiac death (SCD), which can be the first clinical manifestation in ARVC patients.1 Diagnosis of ARVC in children is rare and quite challenging, yet young subjects often have a more severe phenotype with a high risk of SCD.2 While diagnosis of ARVC is currently based on the 2010 revised Task Force Criteria (TFC-2010), it is derived from a predominantly adult population and has not been validated in children.2,3 In particular, certain signs seen in adults, like subtricuspid aneurysms, are hardly seen in children. Moreover, major electrocardiographic criteria described in TFC-2010, including T-wave inversion in leads V1 to V3, are normally found in children and adolescents, and the presence of epsilon waves is rarely seen in children until adolescence. These decrease the diagnostic reliability of electrocardiograms (ECGs) in the pediatric field. Consequently, TFC-2010 often fails to detect ARVC in pediatric patients. In this report, we present 3 patients who had coincidentally undergone echocardiograms several years prior to ARVC diagnosis. We propose a novel, qualitative echocardiographic finding that may be seen early in pediatric patients later diagnosed with ARVC and assess its reliability through 3 case presentations and subsequent case series review of 10 patients with definite ARVC.
Case Presentation 1
A 14-day-old male infant underwent evaluation for a small muscular ventricular septal defect (VSD). The transthoracic echocardiogram (TTE) showed a small VSD and a mushroom-shaped RV outflow tract (RVOT) (ms-RVOT) in the parasternal short axis (PSAX) view but without RV dilation or suspicion for ARVC (Figure 1A, Video 1). Since the VSD closed spontaneously and RV peak longitudinal strain (RVLS, –18.0%) improved compared to early infancy, the patient was discharged from further cardiology follow-up at the age of 2.
Figure 1.
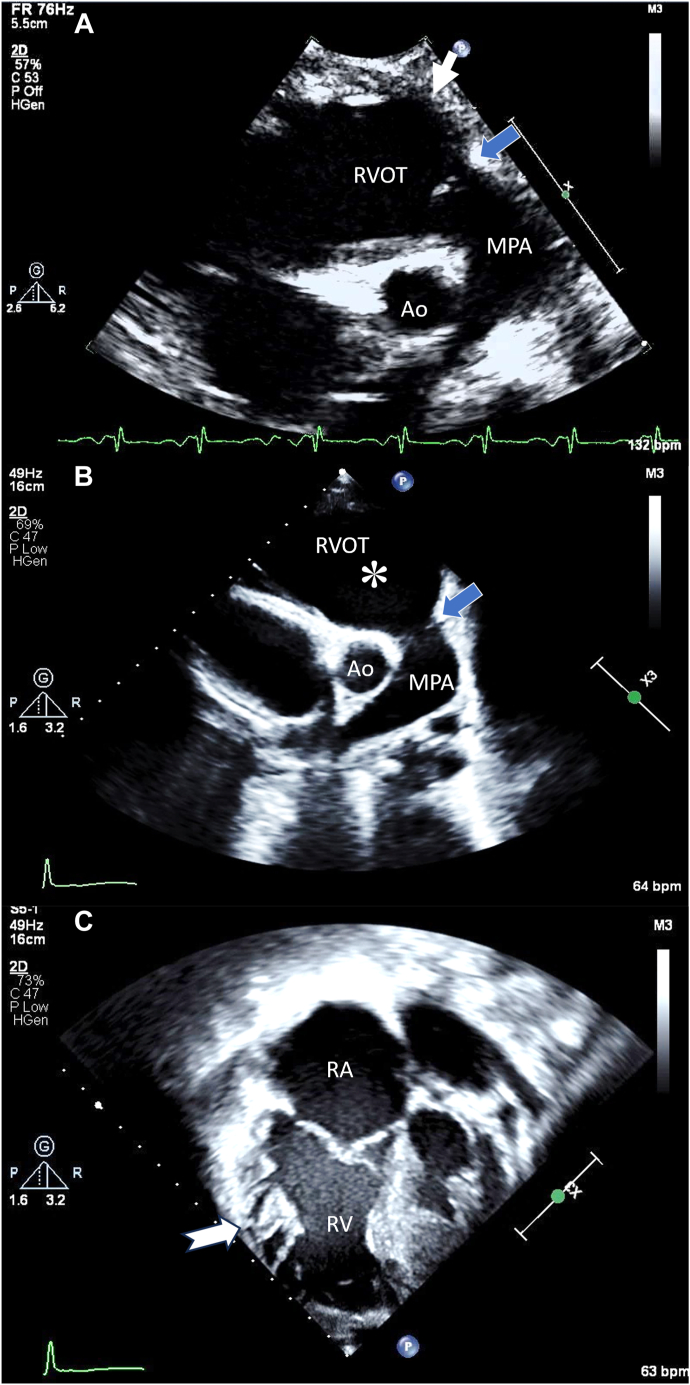
Serial 2D TTE (case 1), PSAX end-diastolic views, demonstrates the ms-RVOT (boxed white arrow; asterisk) and the normal-sized pulmonary annulus (blue arrow) before ARVC diagnosis at age 14 days (A) and during ARVC diagnosis at age 11 years (B). In the apical 4-chamber view, RV dilation and hypertrabeculations along the RV free wall (notched arrow) are noted (C). Ao, Aorta; MPA, main pulmonary artery; RA, right atrium; RV, right ventricle.
After an interval of 9 years, at age 11, the patient presented after resuscitation from ventricular fibrillation–induced cardiac arrest while playing basketball. A TTE showed a progressive RVOT aneurysm, with hypertrabeculations along the RV free wall (Figure 1B and C, Videos 2 and 3), severe RV dilation, and impaired RVLS (–10.2%). Cardiovascular magnetic resonance (CMR) also demonstrated RV dilation (242 mL/m2), regional RV dyskinesia, and a severely reduced RV ejection fraction (RVEF) of 24%. These findings met the major criteria of TFC-2010. Genetic testing confirmed a pathogenic plakophilin-2 (PKP2) gene variant, and an ECG showed diffuse T-wave inversion. The patient was therefore diagnosed with definite ARVC, and an implantable cardioverter-defibrillator (ICD) was placed for secondary prevention. This case exemplifies the presence of an ms-RVOT even in the neonatal period that persisted until the age of diagnosis at 11 years of age.
Case Presentation 2
A 10-year-old boy presented with abnormal ECG and elevated cardiac enzymes following chest pain. The patient’s clinical status deteriorated quickly, culminating in unstable ventricular tachycardia (VT) from presumed acute, fulminant myocarditis, and they were placed on extracorporeal membrane oxygenation. A TTE showed severe biventricular dysfunction without regional wall motion abnormality and an ms-RVOT (Figure 2A, Video 4). Following clinical recovery and decannulation from extracorporeal membrane oxygenation, repeat TTE showed normal left ventricular systolic function prior to discharge from the hospital. A follow-up TTE performed 1 year later showed progression of the RVOT aneurysm (Figure 2B, Video 5) and RV dysfunction (RVLS, –18.5%). However, diagnosis of ARVC was not made at that time, as CMR suggested recurrence of acute myocarditis, rather than ARVC.
Figure 2.
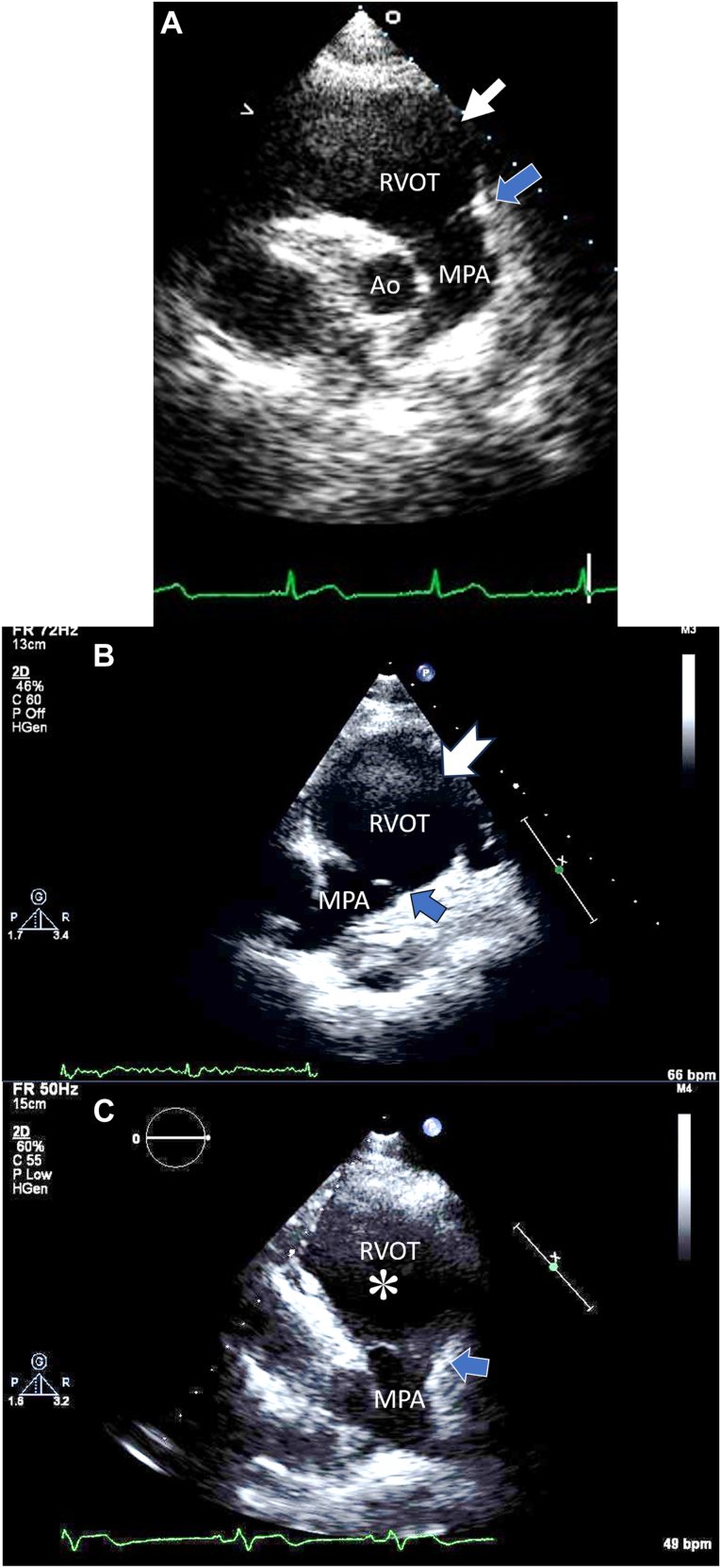
Serial 2D TTE (case 2), PSAX end-diastolic views, demonstrates the ms-RVOT (boxed white arrow; notched arrow; asterisk) and the normal-sized pulmonary annulus (blue arrow) before ARVC diagnosis (A), at 1-year follow-up (B), and after ARVC diagnosis at age 24 years (C). These serial images were captured in slightly different acoustic windows due to aging and sonographer manipulation to avoid lung artifacts. Ao, Aorta; MPA, main pulmonary artery.
During follow-up, RVOT aneurysm and RV function gradually worsened.
At the age of 15, the TTE showed more progression of RVOT dilatation and RV dysfunction (RVLS, –16.8%). Cardiac magnetic resonance also showed severe RV dilation (172 mL/m2) and moderate RV dysfunction (RVEF 41%). Genetic testing did not reveal any mutations, and RV biopsy showed nonspecific findings. Up to 17 years of age, the patient was considered to have RV dysfunction resulting from myocarditis. Echocardiograms during interim follow-up continued to show evidence of an ms-RVOT, but the diagnosis of ARVC was not made.
At the age of 17, the patient presented with hemodynamically unstable VT. Cardiac magnetic resonance continued to show RV enlargement and moderately diminished RV function, consistent with ARVC (Figure 3). At this point, the patient was diagnosed with definite ARVC with additional findings of epsilon waves in the right precordial leads and sustained VT of left bundle-branch morphology with superior axis deviation. The latest TTE at 24 years of age showed progressive RV dilation and RV dysfunction (RVLS, –14%) as well as regional motion abnormalities (Figure 2C, Video 6). It is noteworthy that an ms-RVOT was observed 7 years prior to the diagnosis of ARVC and continued to persist as RV dysfunction progressed.
Figure 3.
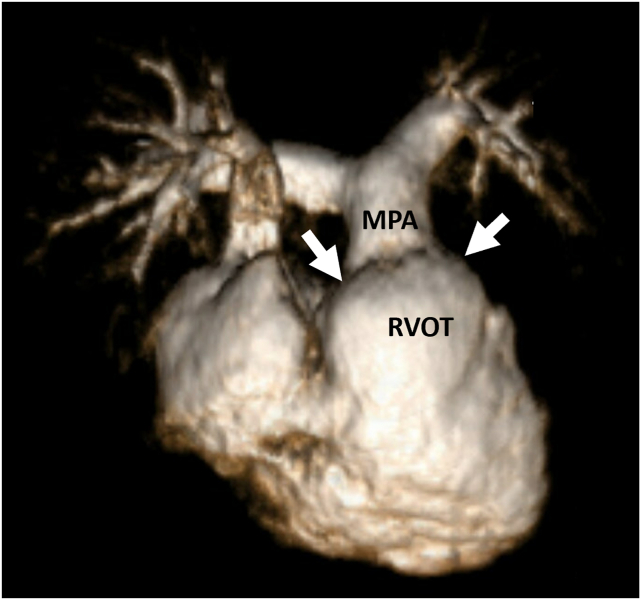
Cardiac magnetic resonance, three-dimensional volume-rendered reconstruction of the right heart, demonstrates RV dilation and the ms-RVOT (arrow) in case 2 at age 17 years. MPA, Main pulmonary artery.
Case Presentation 3
A 15-year-old boy was referred to our institution with acute viral myocarditis. Initial TTE revealed low-normal biventricular function (RVLS, –12.9%) and an ms-RVOT (Figure 4A, Video 7). Cardiac magnetic resonance showed diffusely dyskinetic RV (RVEF 42%) without RV dilation (89.7 mL/m2), consistent with acute myocarditis. During admission, the patient showed gradual recovery of left ventricular function and was discharged with the diagnosis of “resolving myocarditis.”
Figure 4.
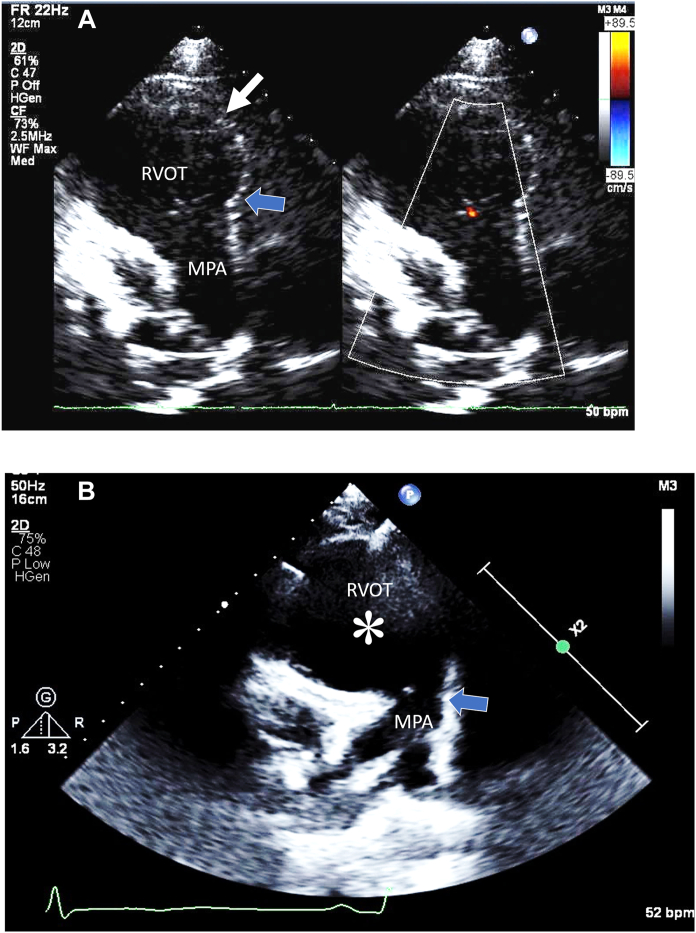
Serial 2D TTE (case 3), PSAX view without (left) and with (right) color-flow Doppler at end-diastole, demonstrates the ms-RVOT (white arrow; asterisk) and the normal-sized pulmonary annulus (blue arrow) before ARVC diagnosis at age 15 years (A) and after ARVC diagnosis at age 17 years (B). MPA, Main pulmonary artery.
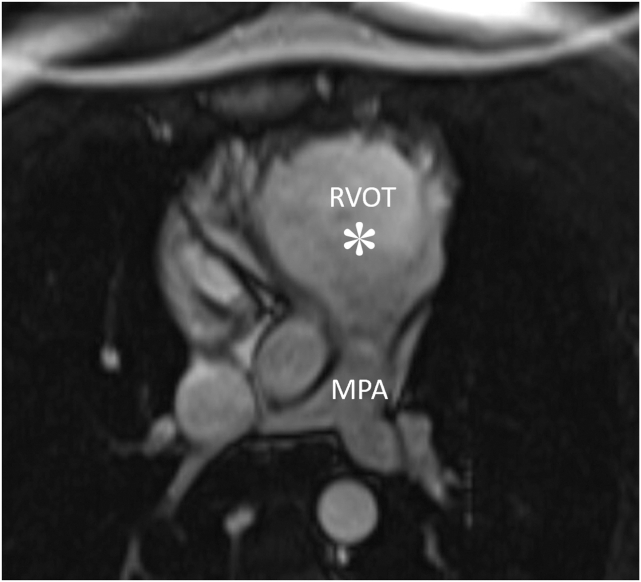
However, the patient experienced recurrent VT within a year of initial presentation. Subsequent genetic testing was positive for a pathogenic mutation in the PKP2 gene. Follow-up CMR showed a locally dyskinetic RV with diminished RVEF of 39%, borderline RV dilation (108.8 mL/m2), and a persistent ms-RVOT (Figure 5), consistent with definite ARVC.
Figure 5.
Cardiac magnetic resonance, balanced steady-state free precession sequence, axial view, demonstrates the ms-RVOT (asterisk) in case 3 within 1 year of TTE. MPA, Main pulmonary artery.
The patient eventually required ICD placement at the age of 17 due to repeated episodes of VT. The latest TTE showed a progressed RVOT aneurysm and RV dilation with diminished RV function (RVLS, –13.0%; Figure 4B, Video 8). Once again, the echocardiographic finding of an ms-RVOT was present early in the disease and continued to be identified during disease progression when other imaging modalities were ambiguous.
Case Series Review
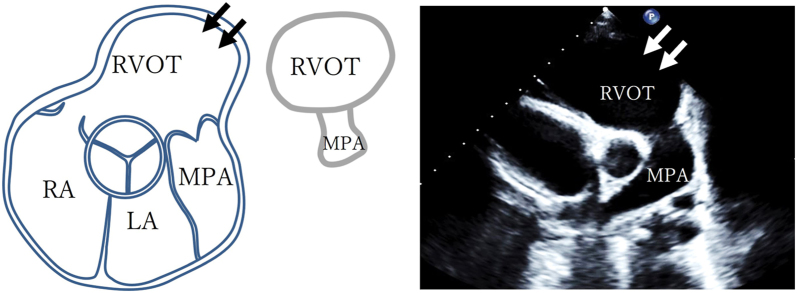
In our case review, another 7 patients fulfilled the diagnostic criteria for definite ARVC according to TFC-2010. The demographic, clinical, and imaging characteristics of patients diagnosed with ARVC based on TFC-2010, including 3 cases mentioned in the earlier case series, are shown in Tables 1 and 2. The median age at diagnosis was 16.5 years. Eight patients were index cases in their family. Seven patients had a pathogenic PKP2 variant, and only 2 patients had a major family history. While 9 (90%) patients met the major criteria of imaging, leading to a definite ARVC diagnosis, only 1 met the major criteria for depolarization abnormalities and arrythmia. Eight (80%) subjects showed an ms-RVOT at diagnosis, which was defined as a significant spherical dilatation of the RVOT with normal-sized pulmonary annulus (Figure 6, Table 2). This characteristic finding of an ms-RVOT was noted in 3 patients even prior to ARVC diagnosis. Table 3 shows the clinical and imaging characteristics of these 3 patients at the first evaluation, during which none of them fulfilled the diagnostic criteria for definite ARVC according to TFC-2010. In these 3 patients, this specific type of RVOT aneurysm persisted with increasing age. Similarly, their RVLS had already declined before diagnosis, and 2 of them exhibited further deterioration with increasing age. Moreover, while 8 (80%) cases exhibited this specific RVOT aneurysm, they also showed mild to moderately decreased RVLS (mean, –15.2% ± 2.8%) at the time of diagnosis.
Table 1.
Clinical characteristics of patients at diagnosis of definite ARVC
| Case | Age, years | Sex | BSA, m2 | Prediagnosis | Gene mutation | ICD placement | TFC-2010 |
Imaging (CMR) |
Endomyocardial biopsy |
Repolarization abnormalities |
Depolarization abnormalities |
Arrhythmia |
Family history of ARVC |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major | Minor | Major | Minor | Major | Minor | Major | Minor | Major | Minor | Major | Minor | Major | Minor | |||||||
| 1 | 12 | M | 1.38 | VSD | PKP2 | Yes | 3 | 1 | +, Echo | - | Not performed | + | – | – | – | – | + | + | – | |
| 2 | 17 | M | 1.89 | Myocarditis | negative | Yes | 3 | 1 | +, Echo | – | – | – | – | + | + | – | + | – | – | – |
| 3 | 18 | M | 1.99 | Myocarditis | PKP2 | Yes | 3 | 1 | +, Echo | – | Not performed | + | – | – | – | – | + | + (Mother) | – | |
| 4 | 15 | M | 2.38 | VT | PKP2 | Yes | 2 | 2 | +, Echo | – | Not performed | – | – | – | + | – | + | + | – | |
| 5 | 17 | F | 1.95 | NA | PKP2 | Yes | 3 | 1 | + | Echo | Not performed | + | – | – | – | – | + | + | – | |
| 6 | 16 | M | 1.87 | NA | PKP2 | Yes | 3 | 2 | +, Echo | – | Not performed | + | – | – | + | – | + | + | – | |
| 7 | 15 | M | 2.04 | NA | PKP2 | Yes | 3 | 0 | +, Echo | – | Not performed | + | – | – | – | – | – | + | – | |
| 8 | 18 | M | 1.97 | VT | LDB3 | Yes | 1 | 2 | –, Echo | – | Not performed | – | + | – | – | – | + | – | – | |
| 9 | 18 | F | 1.99 | NSVT | PKP2 | No | 1 | 2 | – | – | Not performed | – | + | – | – | – | + | + (Mother) | – | |
| 10 | 13 | M | 1.33 | NA | NA | No | 2 | 1 | +, Echo | – | Not performed | + | – | – | – | – | + | – | – | |
BSA, body surface area; F, female; M, male; NA, not applicable; NSVT, nonsustained VT.
Table 2.
Imaging characteristics of patients at diagnosis of definite ARVC
| Case | Echocardiography |
CMR |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RVOT distal |
PLAX |
PSAX |
PA annulus |
RV function |
RVLS |
ms-RVOT | Subtricuspid aneurysm | Hypertrabeculations | LVEF, % | RVEDVI, mL/m2 | RVEF, % | ||||||
| Diameter, mm | To BSA, mm/m2 | Diameter, mm | To BSA, mm/m2 | Diameter, mm | To BSA, mm/m2 | Diameter, mm | Z score | RVFAC, % | Whole (%) | Free wall, % | |||||||
| 1 | 51.3 | 31.2 | 46.6∗ | 33.8∗ | 44.6∗ | 32.3∗ | 25.3 | 0.15 | 33.7† | –10.2 | –12.1 | Yes | – | + | 62 | 242∗ | 24∗ |
| 2 | 49.0 | 25.9 | 51.5∗ | 27.2∗ | 52.8∗ | 27.9∗ | 29.1 | 0.36 | 26.9∗ | –16.2 | –16.0 | Yes | + | + | 64 | 170∗ | 39∗ |
| 3 | 43.7 | 22.0 | 40.8∗ | 20.5∗ | 40.5∗ | 20.4† | 25.6 | –0.53 | 25.6∗ | –13.0 | –13.8 | Yes | – | – | 59 | 109† | 39∗ |
| 4 | 40.8 | 17.1 | 43.0∗ | 18.1† | 43.5∗ | 18.3† | 32.4 | 0.30 | 31.4∗ | –14.6 | –14.3 | Yes | – | + | 59 | 139∗ | 40∗ |
| 5 | 35.4 | 18.2 | 31.8† | 16.3† | 31.0 | 15.9 | 24.4 | –0.73 | 36.5† | –19.7 | –21.2 | Yes | – | – | 64 | 113∗ | 45† |
| 6 | 56.3 | 30.1 | 41.2∗ | 22.0∗ | 51.3∗ | 27.4∗ | 25.7 | –0.34 | 27.0∗ | –17.1 | –16.6 | Yes | – | – | 66 | 202∗ | 35∗ |
| 7 | 43.6 | 21.4 | 46.9∗ | 23.0∗ | 44.2∗ | 21.7∗ | 27.3 | –0.24 | 29.8∗ | –14.4 | –15.6 | Yes | – | – | 62 | 169∗ | 39∗ |
| 8 | 26.6 | 13.5 | 35.9∗ | 18.2∗ | 37.0∗ | 18.8† | 23.1 | –1.04 | 36.3† | –18.8 | –19.7 | No | – | – | 57 | 94 | 52 |
| 9 | 26.1 | 13.1 | 28.2 | 14.2 | 30.9 | 15.5 | 28.0 | –0.03 | 54.0 | –23.6 | –25.1 | No | – | – | 65 | 65 | 78 |
| 10 | 35.5 | 26.7 | 40.2∗ | 30.2∗ | 36.0∗ | 27.1∗ | 23.6 | 0.17 | 27.0∗ | –16.2 | –18.7 | Yes | – | – | 49 | 122∗ | 40∗ |
BSA, body surface area; FAC, fractional area change; LVEF, left ventricular ejection fraction; PA, pulmonary artery; PLAX, parasternal long-axis view; RV, right ventricle; RVEDVI, RV end-diastolic volume index.
Meets major criteria by TFC-2010.
Meets minor criteria by TFC-2010.
Figure 6.
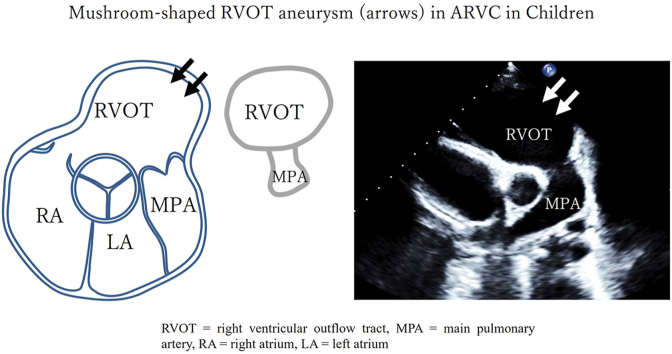
A schematic diagram depicts a characteristic ms-RVOT (arrows) in a PSAX display adjacent to a diagram of a mushroom with echo labels for illustrative pattern recognition. MPA, main pulmonary artery; LA, left atrium; RA, right atrium.
Table 3.
Clinical and Imaging characteristics of 3 patients prior to diagnosis of ARVC
| Case∗ | Age | Sex | Ht, cm | BW, kg | BSA, m2 | TFC-2010 category | Echocardiography |
CMR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RVOT distal to BSA, mm/m2 | PLAX to BSA, mm/m2 | PSAX to BSA, mm/m2 | PA annulus |
RV function: RVFAC, % | RVLS, % |
LVEF, % | RVEDVI, mL/m2 | RVEF, % | |||||||||
| Diameter, mm | Z score | Whole, % | Free wall, % | ||||||||||||||
| 1 | 14 days | Male | 51 | 4.3 | 0.25 | No | 84.8 | 71.2† | 71.6† | 10.8 | 0.44 | 21.2† | –13.3 | –14.2 | 64 | – | – |
| 2 | 15 years | Male | 172 | 61.7 | 1.71 | Possible | 27.3 | 26.0† | 26.8† | 23.0 | –0.71 | 28.1† | –16.8 | –18.5 | 61 | 172† | 41‡ |
| 3 | 15 years | Male | 178 | 72.0 | 1.89 | Borderline | 25.0 | 19.2† | 19.2‡ | 28.2 | 0.17 | 29.9† | –12.9 | –13.7 | 48 | 90 | 42‡ |
BSA, body surface area; BW, body weight; FAC, fractional area change; Ht, Height; LV, left ventricle; LVEF, left ventricular ejection fraction; PA, pulmonary artery; PLAX, parasternal long-axis view; RV, right ventricle; RVEDVI, RV end-diastolic volume index.
Meets major criteria by TFC-2010.
Meets minor criteria by TFC2010.
In an effort to demonstrate the validity of our qualitative finding, an expert pediatric echocardiographer (M.Q.) performed a blinded review of patients with ARVC and age-matched controls and was able to consistently detect ms-RVOT from the PSAX views in each of the patients.
Discussion
This case series reports the clinical characteristics and specific echocardiographic findings in pediatric-onset ARVC. Notably, the majority of patients with definite ARVC had a unique finding of an ms-RVOT. Some patients had this finding even before the diagnosis of ARVC was made. However, due to lack of familiarity with the association of this finding to ARVC, its presence in these 3 pediatric patients did not lead to a diagnosis of this rare disease.
Diagnosis of ARVC before adolescence can be challenging, with significant limitations of TFC-2010 in the pediatric age group. Because there is an increased risk of SCD in pediatric-onset ARVC, especially in probands,1 it is of paramount importance that this disease be diagnosed early in children so that appropriate measures, such as the use of ICD and activity restrictions for primary prevention of SCD, can be considered. Cardiac magnetic resonance is believed to be the most accurate imaging modality for the diagnosis of ARVC.2 Nevertheless, we should be cognizant that the first imaging test performed in most pediatric patients is invariably an echocardiogram. Since the chest wall of a child is much thinner than that of an adult, the RVOT is extremely close to the transducer in children. This results in an improper offset between the transducer and RVOT, producing a near-field clutter artifact. This makes measurements of RVOT proposed by TFC-2010 difficult in children. Gotschy et al.4 reported that the distal RVOT diameter defined by the PSAX view exhibited the poorest correlation with CMR. Pieles et al.5 showed that echocardiographic findings of TFC-2010 are not met by a majority of adolescent ARVC patients. While they demonstrated that RVLS and end-diastolic diameter at the apical third of the RV were significantly associated with ARVC, these quantitative measurements are not performed routinely in other laboratories. In line with this report by Pieles et al., our case series also revealed that 90% of patients with definite ARVC exhibited a deterioration in RVLS at the time of diagnosis. It is notable that decreased RVLS may occur early in the disease process (as noted in case 1). While decreased RV systolic function may also be seen in isolated RV myocarditis and Uhl disease in children, these diseases have different presentations and laboratory markers, and none show the ms-RVOT that we described in children with ARVC.6,7
In our case series, we opted not to focus on RV strain, as it is not universally performed. Instead, we wished to propose a simple two-dimensional (2D) echocardiographic feature, which can be easily detected and serve as a qualitative warning to readers of pediatric echocardiograms.
This qualitative finding of a ms-RVOT could serve as an initial clue to a pediatric echocardiographer and should trigger a close longitudinal follow-up of patients without any symptoms or family history. The follow-up should include quantitative evaluations with CMR and RV strain measurement, which may lead to early detection of ARVC. Furthermore, it is important that the ms-RVOT should be evaluated only in the PSAX view, as the parasternal long-axis view of RVOT may produce false-positive findings in children. The apical orientation of the heart is more horizontal in children because the diaphragm is in a more horizontal position compared to adults.8 Therefore, a standard parasternal long-axis view that is tangential to the RVOT may include more of the RVOT in children than in adults, producing overestimation of RVOT size.
Finally, in a patient with RV dilation without explanation, one should investigate whether there is also dilation of the pulmonary annulus or main pulmonary artery. Patients with ARVC exhibit a rather characteristic RVOT dilation, where the pulmonary annulus and main pulmonary artery are not dilated. Previous studies in adults and children have shown that RV dilation in ARVC patients is characterized by fibrofatty replacement of RV myocardium, resulting in thinning and aneurysm formation in the RVOT. The pulmonary annulus consists of tight collagenous tissue, which merges with the fibroelastic walls of the main pulmonary artery. Since there is no myocardium in the latter 2 structures, the fibrofatty invasion of the RV does not affect the pulmonary valve annulus and main pulmonary artery.9 The sparing of the pulmonary annulus and main pulmonary artery contributes to the development of a typical ms-RVOT in patients with ARVC.
Genetic analysis in our case review showed that 70% of patients carried the PKP2 pathogenic variant. Genotype-phenotype correlation studies in adults have reported the PKP2 variant to be associated with a higher incidence of RV involvement, especially RVOT aneurysms.10 We speculate that the presence of PKP2 variant in children may be associated with the formation of an ms-RVOT. Due to a limited number of patients, we can merely point out this association but cannot comment on any causal role of this genetic variant.
When RV dilation is detected in children, it is essential to exclude subtle congenital heart diseases that may cause RV volume overload, such as an atrial septal defect, an unroofed coronary sinus, or partial anomalous pulmonary venous connection. However, none of these lesions produce ms-RVOT.
Even if the other findings in the 2010-TFC are not demonstrated in children, presence of ms-RVOT is an important clue to consider progression to definite ARVC. We speculate that an ms-RVOT observed in children may evolve in shape with growth into a more diffuse dilatation with increasing age, as noted in adults.
Conclusion
Pediatric-onset ARVC may be suspected in early stages before progression to definite ARVC by the qualitative finding of an ms-RVOT. This finding may serve as an early clue that may raise caution and urge closer monitoring of these patients, even in the absence of symptoms or family history. This finding has not been previously reported in children or young adults. Its presence may trigger preemptive management strategies, including close monitoring to prevent SCD.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2024.03.003.
Supplementary Data
Serial 2D TTE, PSAX view, demonstrates the ms-RVOT and the normal-sized pulmonary annulus before ARVC diagnosis in case 1 at age 14 days.
Serial 2D TTE, PSAX view, demonstrates the larger ms-RVOT and the normal-sized pulmonary annulus during ARVC diagnosis in case 1 at age 11 years.
Serial 2D TTE, apical 4-chamber view, demonstrates hypertrabeculations along the dilated, dysfunctional RV free wall during ARVC diagnosis in case 1 at age 11 years.
Two-dimensional TTE, PSAX view, demonstrates the ms-RVOT and the normal-sized pulmonary annulus in case 2 before ARVC diagnosis.
Serial 2D TTE, PSAX view, demonstrates a larger ms-RVOT and a normal-sized pulmonary annulus in case 2, after baseline TTE but before ARVC diagnosis.
Serial 2D TTE, PSAX view, demonstrates the ms-RVOT and normal-sized pulmonary annulus in case 2 after ARVC diagnosis.
Two-dimensional TTE, PSAX view without (left) and with (right) color-flow Doppler, demonstrates the ms-RVOT and mild pulmonary regurgitation in case 3 before ARVC diagnosis.
Serial 2D TTE, PSAX view, demonstrates a larger ms-RVOT and a normal-sized pulmonary annulus in case 3 after ARVC diagnosis.
References
- 1.Roudijk R.W., Verheul L., Bosman L.P., Bourfiss M., Breur J., Slieker M.G., et al. Clinical characteristics and follow-up of pediatric-onset arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol. 2022;8:306–318. doi: 10.1016/j.jacep.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Te Riele A., James C.A., Calkins H., Tsatsopoulou A. Arrhythmogenic right ventricular cardiomyopathy in pediatric patients: an important but underrecognized clinical entity. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.750916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotschy A., Saguner A.M., Niemann M., Hamada S., Akdis D., Yoon J.N., et al. Right ventricular outflow tract dimensions in arrhythmogenic right ventricular cardiomyopathy/dysplasia-a multicentre study comparing echocardiography and cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2018;19:516–523. doi: 10.1093/ehjci/jex092. [DOI] [PubMed] [Google Scholar]
- 5.Pieles G.E., Grosse-Wortmann L., Hader M., Fatah M., Chungsomprasong P., Slorach C., et al. Association of echocardiographic parameters of right ventricular remodeling and myocardial performance with modified task force criteria in adolescents with arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.118.007693. [DOI] [PubMed] [Google Scholar]
- 6.Faria B., von Hafe P., Ferreira F.C., Almeida F., Dias G., Cardoso F., et al. Uhl’s anomaly: 10 Years of follow-up of an unoperated patient. CASE (Philadelphia, Pa) 2020;4:351–355. doi: 10.1016/j.case.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Driss A., Laissy J.-P. Acute isolated right ventricular myocarditis: the importance of multimodality imaging. Circ Cardiovasc Imaging. 2021;14 doi: 10.1161/CIRCIMAGING.121.013046. [DOI] [PubMed] [Google Scholar]
- 8.Di Cicco M., Kantar A., Masini B., Nuzzi G., Ragazzo V., Peroni D. Structural and functional development in airways throughout childhood: children are not small adults. Pediatr Pulmonol. 2021;56:240–251. doi: 10.1002/ppul.25169. [DOI] [PubMed] [Google Scholar]
- 9.Misfeld M., Sievers H.H. Heart valve macro- and microstructure. Philos Trans R Soc Lond B Biol Sci. 2007;362:1421–1436. doi: 10.1098/rstb.2007.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pamuru P.R., Dokuparthi M.V.N., Remersu S., Calambur N., Nallari P. Comparison of Uhl’s anomaly, right ventricular outflow tract ventricular tachycardia (RVOT VT) & arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) with an insight into genetics of ARVD/C. Indian J Med Res. 2010;131:35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial 2D TTE, PSAX view, demonstrates the ms-RVOT and the normal-sized pulmonary annulus before ARVC diagnosis in case 1 at age 14 days.
Serial 2D TTE, PSAX view, demonstrates the larger ms-RVOT and the normal-sized pulmonary annulus during ARVC diagnosis in case 1 at age 11 years.
Serial 2D TTE, apical 4-chamber view, demonstrates hypertrabeculations along the dilated, dysfunctional RV free wall during ARVC diagnosis in case 1 at age 11 years.
Two-dimensional TTE, PSAX view, demonstrates the ms-RVOT and the normal-sized pulmonary annulus in case 2 before ARVC diagnosis.
Serial 2D TTE, PSAX view, demonstrates a larger ms-RVOT and a normal-sized pulmonary annulus in case 2, after baseline TTE but before ARVC diagnosis.
Serial 2D TTE, PSAX view, demonstrates the ms-RVOT and normal-sized pulmonary annulus in case 2 after ARVC diagnosis.
Two-dimensional TTE, PSAX view without (left) and with (right) color-flow Doppler, demonstrates the ms-RVOT and mild pulmonary regurgitation in case 3 before ARVC diagnosis.
Serial 2D TTE, PSAX view, demonstrates a larger ms-RVOT and a normal-sized pulmonary annulus in case 3 after ARVC diagnosis.