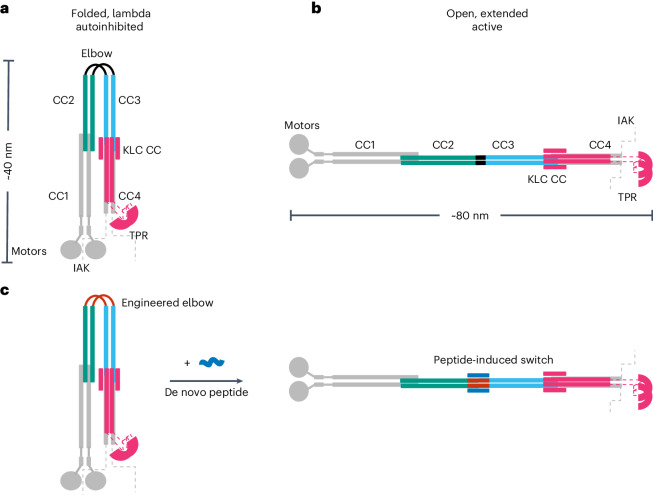
Fig. 1. Architecture of the autoinhibited and active states of kinesin-1 and target design.
a,b, Schematic illustrating the autoinhibited lambda particle (a) and open conformer (b) of heterotetrameric kinesin-1. Globular (motor and tetratricopeptide repeat (TPR)) domains are labeled and the coiled-coil domains of the KHCs are numbered CC1–CC4, as previously described28. KHCs are shown with motor domains (motors) and coiled-coil domains 1 and 4 (CC1 and CC4) in gray. Gray dashed lines indicate the unstructured KHC C-terminal tails that contain the IAK regulatory motif. The key coiled-coil domains discussed in this study are highlighted in teal (CC2) and cyan (CC3). The elbow between CC2 and CC3 is in black. KLCs are shown in magenta, indicating the six-helix bundle predicted at the KHC–KLC28 interface, with dashed lines indicating the unstructured linker to the globular TPR domains involved in cargo recognition. c, The aim of this work is to engineer a de novo peptide (red) into the elbow of a lambda state to construct a peptide-inducible (blue) conformational switch to the open state.